A diblock fusion protein with dual functions of adhesion and antifreeze, its synthesis method and application
A fusion protein and synthesis method technology, applied in the biological field, can solve the problems of cumbersome operation, cumbersome preparation method of superhydrophobic material, protein inactivation, etc., and achieves the effects of low equipment requirements, easy later production expansion, and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Recombinant expression vector and strain
[0040] 1 Recombinant expression vector
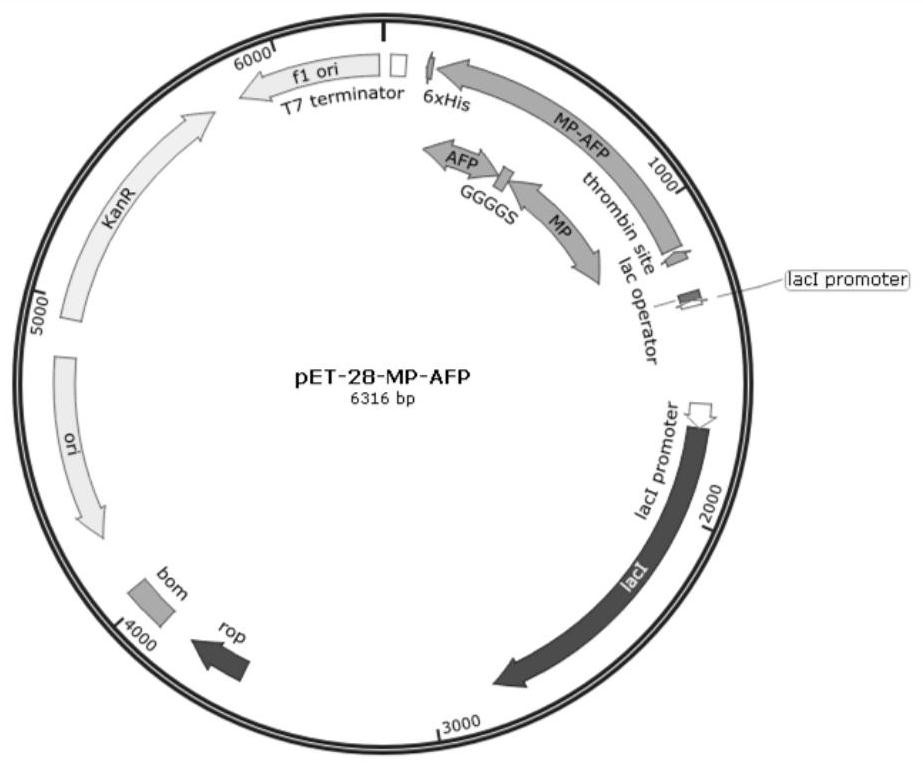
[0041] The expression of fusion protein MP-AFP is configured to connect to a polypeptide to connect to a polypeptide to connect to a polypeptide. The 5 'end of the gene sequence is added to the BamHI restriction enzyme digestion site and the NOTI restriction enzyme disaster is added; the manual synthesis of the gene sequence and cloned into E. coli expression vector PET-28A to obtain recombinant expression vectors PET-28A-MP-AFP containing T7 strong label, LCA lactose, kanamycin resistance marker site, and hexamemonic label, such as the recombinant expression vector. figure 1 Indicated.
[0042] 2 chemical transformation of E. coli
[0043] The substance E. coli BL21 (DE3) is melted on ice. The correct recombinant expression vector PET-28A-MK-AFP will be constructed, and the expression vector of the expression tyrosinase is mixed with the sensitudinal E. coli BL21 (DE3), incubated...
Embodiment 2
[0044] Example 2: Expression purification of fusion protein MP-AFP
[0045] 1 Escherichia coli fermentation expression fusion protein MP-AFP
[0046] E. coli converted into transformed E. coli is activated on a double-resistant LB solid medium containing kanamycin and chloramphenicol. Picking with a monomida Follow 5 ml of kanamycin containing 50 μg / ml of lt liquid medium, at 37 ° C, 200 rpm shock and cultured overnight. Press 1: 100 ratio to the 500 ml shake flask containing 200 ml of medium; the growth curve of E. coli is measured with an UV spectrophotometer, and the concentration of the bacterial liquid is OD after E. coli. 600 At 0.8 mm, E. coli was induced by 0.8 mM IPTG, and E. coli was collected after 12 h, 250 rpm.
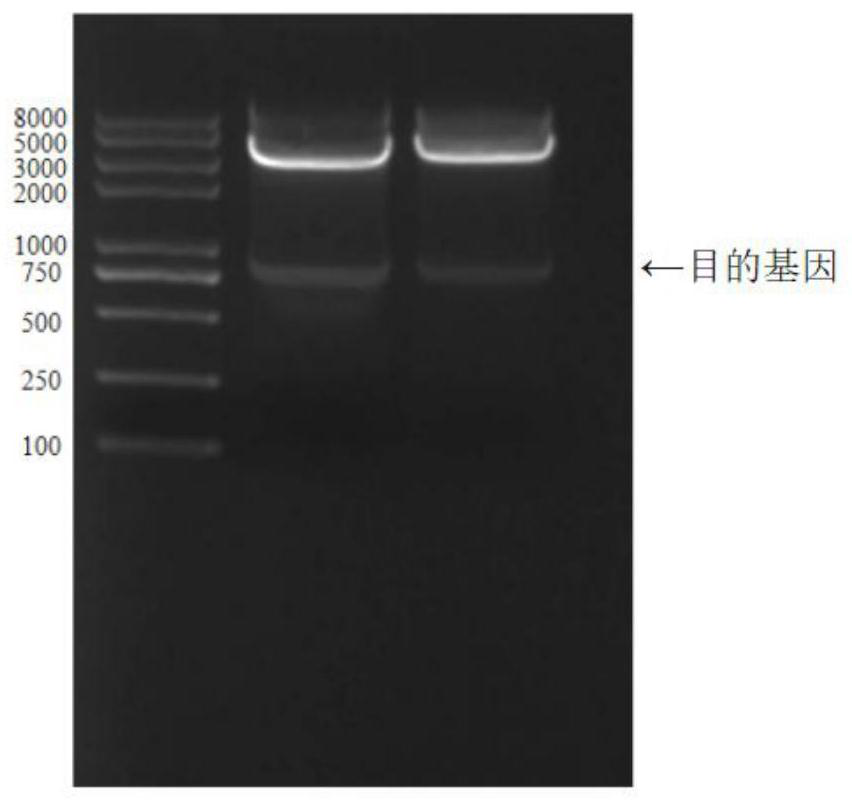
[0047] 2 separation and purification of fusion protein MP-AFP
[0048] Escherichia coli fermentation broth was centrifuged at 4 ° C, 6000 rpm. The primary bacterium is resuspended with a PBS solution, the bacterial liquid is ultrasound under the ice water b...
Embodiment 3
[0054] Example 3: Surface modification and atomic force microscopy analysis
[0055] 1 Sample surface modification of the matrix material
[0056] The glass sheet and the mica were matrix material, the surface of the sample modified matrix material with water and fusion protein MP-AFP. The cleansing of the cleansing matrix material was infiltrated in two samples at 25 ° C for 12 h at 25 ° C, and at least 3 repetitions per group were incubated at 25 ° C. After the incubation, the matrix material was washed 3 times with high purity water to remove excess protein samples.
[0057] 2 atomic force microscope detection
[0058] The surface morphology of the matrix material is detected by the atomic force microscope, and the surface of the fusion protein modified glass sheet is compactly dense protein trimming layer, and the regular order.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com