Injectable hydrogel and preparation method thereof
A technology for injecting water and gel, which is applied in the fields of biomedical materials and tissue engineering, can solve the problems of uncontrollable degradation rate, complicated operation, and destruction of three-dimensional matrix cross-linked network, etc., and achieve the effect of controllable degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
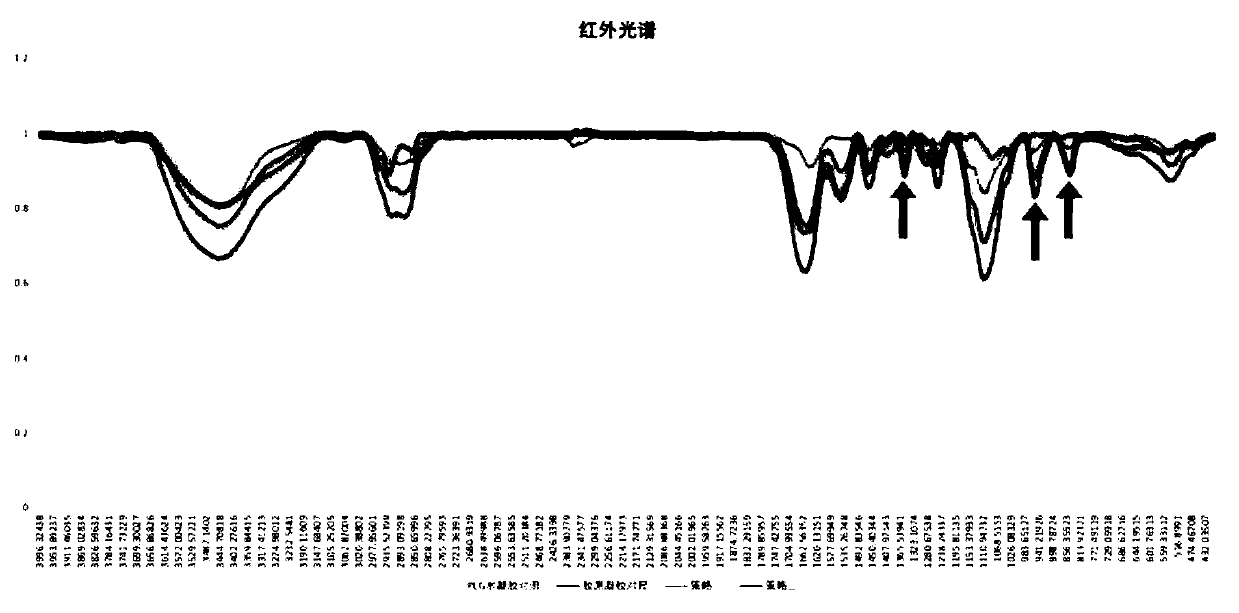
Image
Examples
Embodiment 1
[0033] A preparation method of injectable hydrogel, comprising the following steps:
[0034] (1) The adipose tissue was repeatedly frozen and thawed 3 times, then soaked in 0.4mol / L NaCl hypertonic liquid for 4 hours, and then soaked in 0.9mol / L NaCl for 4 hours to break the fat cells; The trypsin solution was digested at 35°C for 3 hours, washed with deionized water for 3 hours, treated with isopropanol for 12 hours to remove fat, and then treated with 0.9 wt% sodium lauryl sulfate for 2 hours, and then washed with 0.9% polyethylene glycol octylphenyl ether was treated for 48 hours to perform decellularization to obtain an acellular matrix, which was freeze-dried at minus 80°C and 0.038mbar for 36 hours for use;
[0035] (2) Enzymolyze the lyophilized acellular matrix with 0.5 mg / mL pepsin in an acidic environment for 12 hours, then inactivate the pepsin to obtain a 6 mg / mL extracellular matrix protein solution for use;
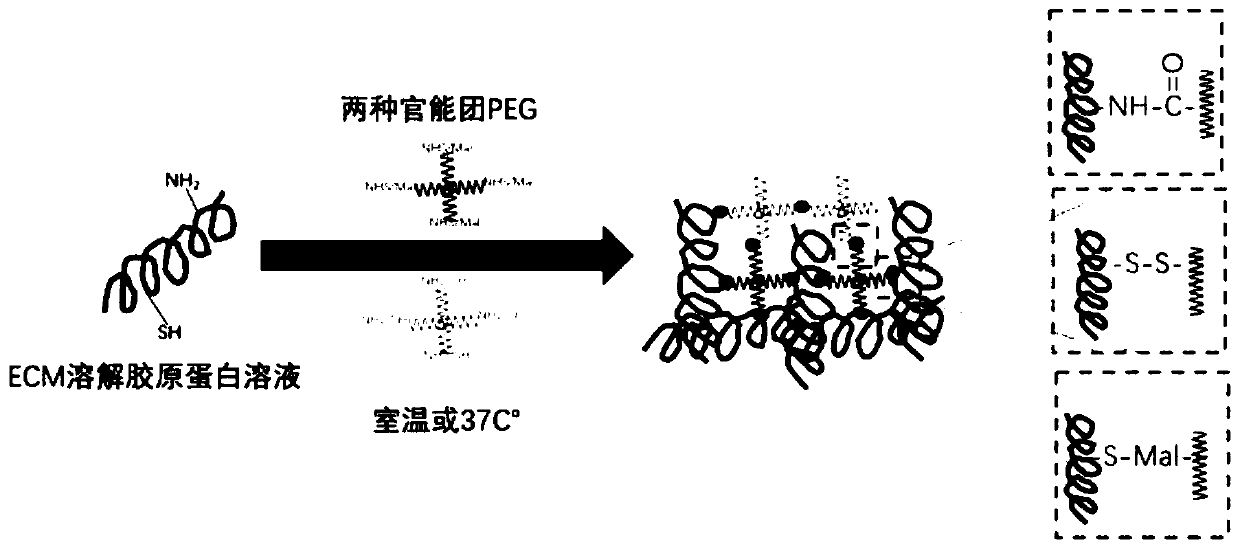
[0036] (3) The extracellular matrix protein solution ...
Embodiment 2
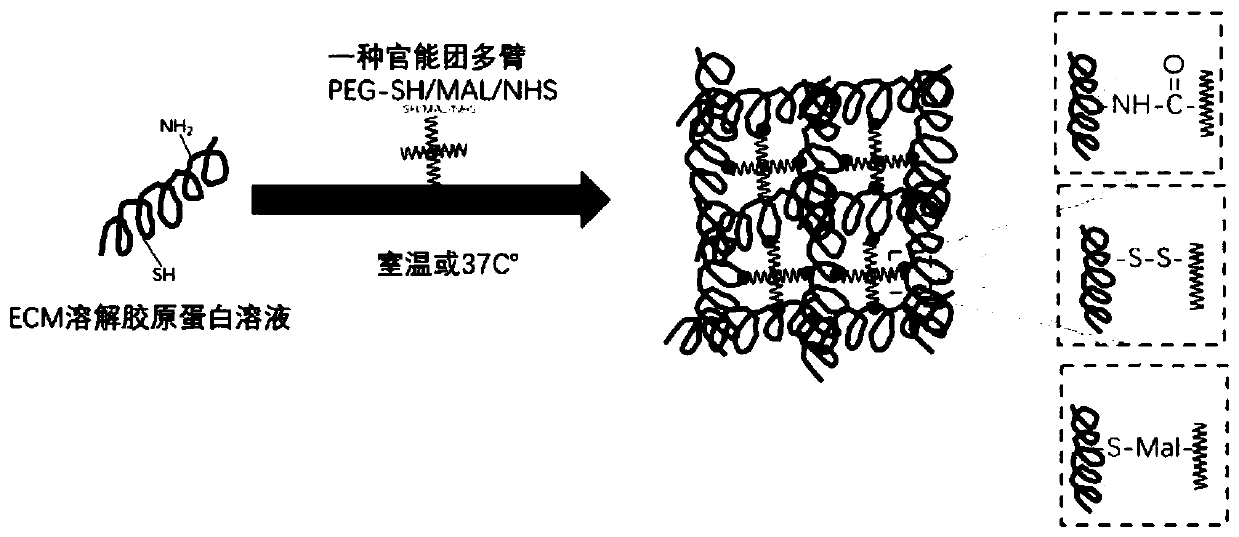
[0038] Such as figure 1 Shown, a kind of preparation method of injectable hydrogel comprises the following steps:
[0039] (1) The adipose tissue was repeatedly frozen and thawed 4 times, then soaked in 0.5mol / L NaCl hypertonic liquid for 4 hours, and then soaked in 1mol / L NaCl for 4 hours to break the fat cells; The protease solution was digested at 37°C for 6 hours, washed with deionized water for 4 hours, treated with isopropanol for 18 hours to remove fat, and then treated with 1wt% sodium lauryl sulfate for 18 hours, followed by 1% The polyethylene glycol octyl phenyl ether was treated for 48 hours to perform decellularization to obtain an acellular matrix, which was freeze-dried at minus 80°C and 0.04mbar for 36 hours for use;
[0040] (2) enzymatically hydrolyzing the lyophilized acellular matrix with 3 mg / mL pepsin in an acidic environment for 18 hours, and then inactivating the pepsin to obtain a 20 mg / mL extracellular matrix protein solution for use;
[0041] (3) C...
Embodiment 3
[0043] A preparation method of injectable hydrogel, comprising the following steps:
[0044] (1) The adipose tissue was repeatedly frozen and thawed for 6 times, then soaked in 0.6mol / L NaCl hypertonic liquid for more than 4 hours, and then soaked in 1.1mol / L NaCl for 5 hours to break the fat cells; then use 0.26wt% The trypsin solution was digested at 38°C for 10 hours, washed with deionized water for 4 hours, treated with isopropanol for 24 hours to remove fat, and then treated with 1.1wt% sodium lauryl sulfate for 24 hours. Treat with 1.1% polyethylene glycol octylphenyl ether for 50 hours to perform decellularization treatment to obtain an acellular matrix, and freeze-dry the acellular matrix at minus 80°C and 0.042mbar for 38 hours for use;
[0045] (2) enzymatically hydrolyzing the lyophilized acellular matrix with 5 mg / mL pepsin in an acidic environment for 24 hours, and then inactivating the pepsin to obtain a 40 mg / mL extracellular matrix protein solution for use;
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com