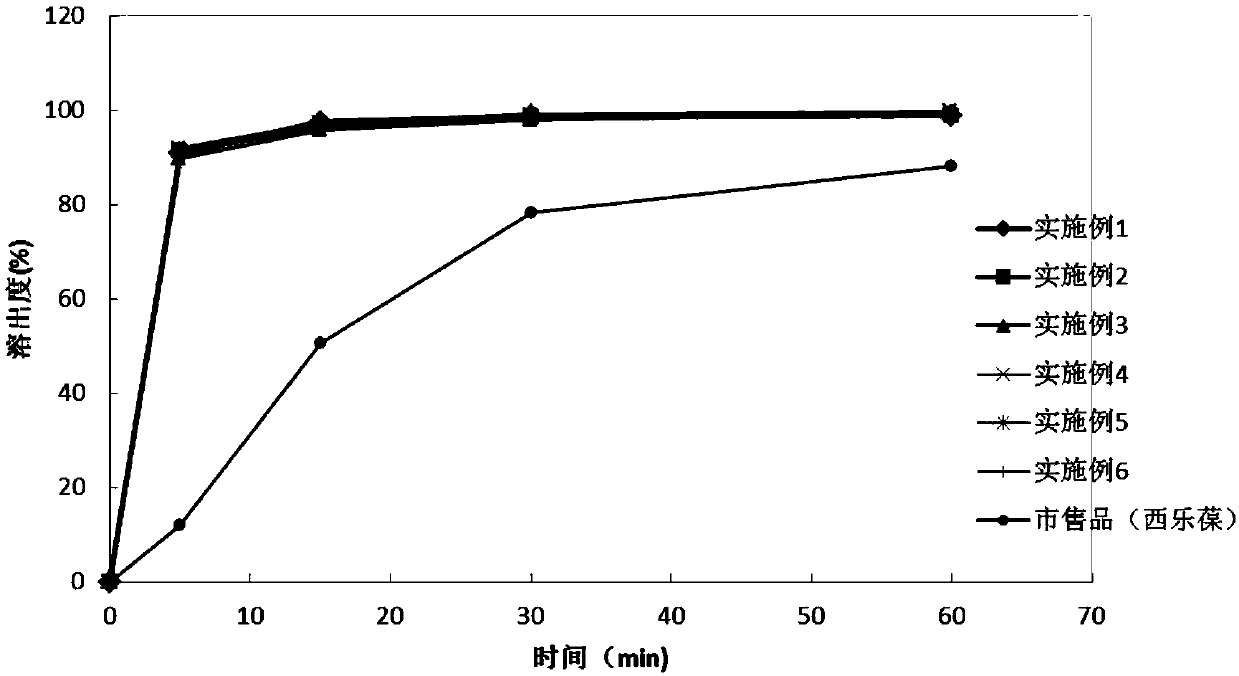
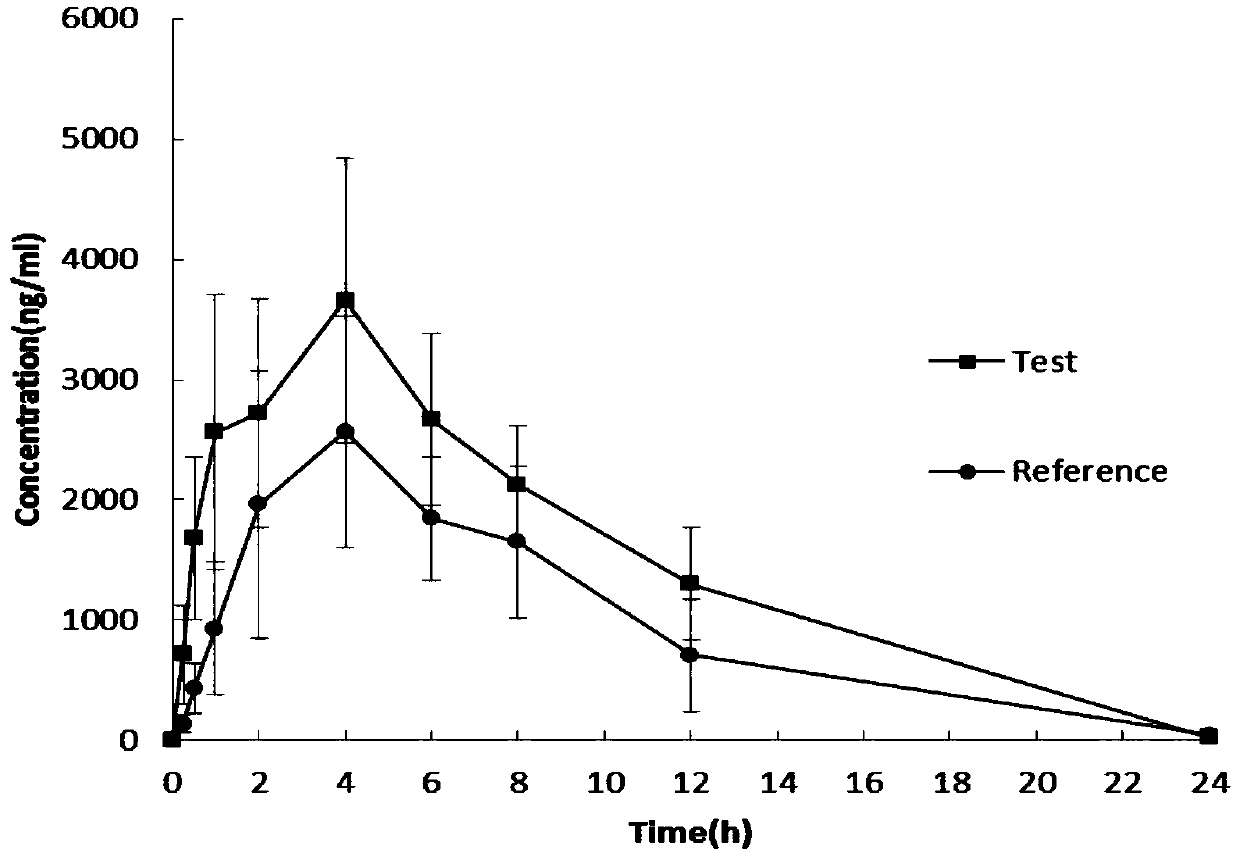
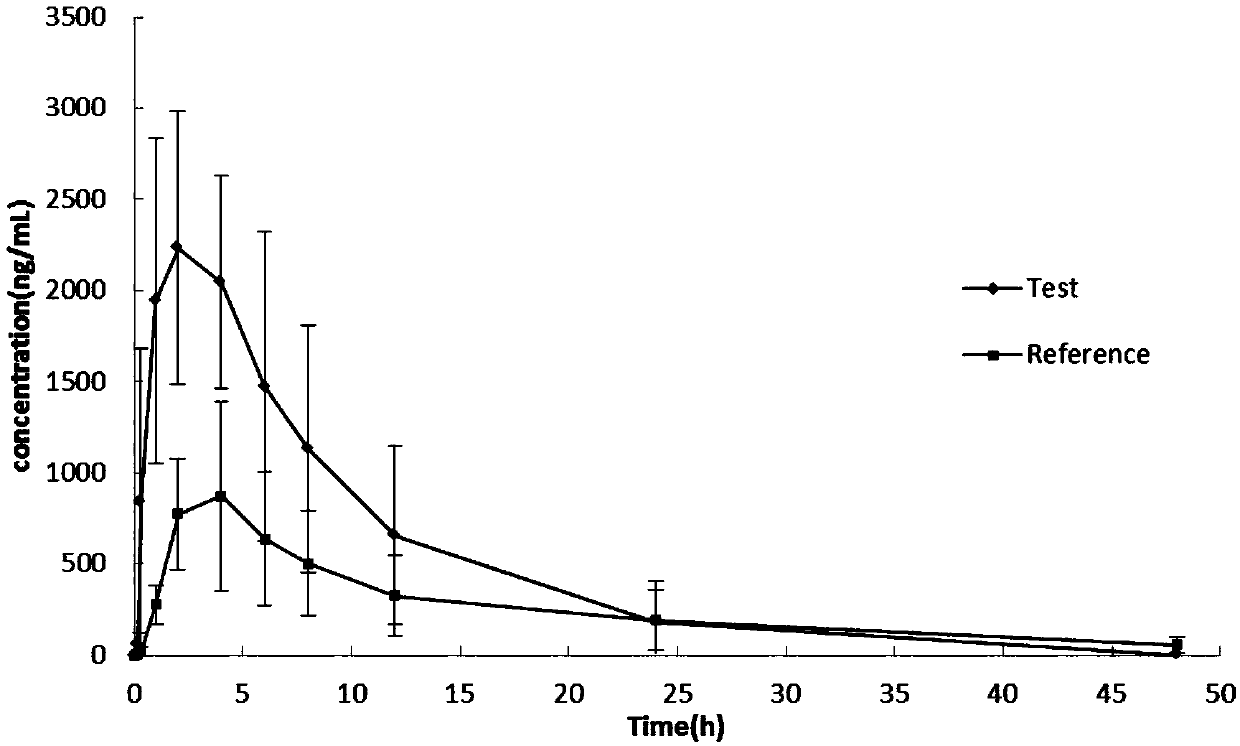
Celecoxib lyophilized orally disintegrating tablets with high bioavailability and preparation method thereof
A celecoxib, utilization technology, applied in the field of celecoxib freeze-dried orally disintegrating tablets and its preparation, can solve the difficult problems of solvent residue, low bioavailability, low oral compliance, etc., to reduce adverse reactions Risk of reactions, reduction of local irritation, effect of low doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Recipe composition:
[0029]
[0030] The specific preparation method is: adding polyvinylpyrrolidone and sodium lauryl sulfate into 200mL of pure water and stirring to dissolve. Slowly pour the celecoxib raw material into the dispersion medium and stir for 5 minutes, so that the celecoxib is fully wetted and dispersed in the dispersion medium to obtain an initial suspension. Add the above primary suspension into a nano grinder, adjust the rotation speed to 3000 rpm, and wet grind for 30 minutes to obtain a nanoscale drug suspension. Add the prescribed amount of mannitol and aspartame into the suspension, stir and mix until fully dissolved, and obtain a suspension for freeze-drying. After vacuum degassing the above suspension, inject it into the mold accurately. Freeze-dry the mold filled with the liquid medicine according to the freeze-drying curve: Pre-freeze at -100°C for 2 hours - vacuumize - maintain at -40°C for 3 hours - maintain at -20°C for 8 hours - maint...
Embodiment 2
[0032] Recipe composition:
[0033]
[0034]
[0035] Add polyvinylpyrrolidone and sodium lauryl sulfate into 200 mL of pure water and stir to dissolve. Slowly pour the celecoxib raw material into the dispersion medium and stir for 5 minutes, so that the celecoxib is fully wetted and dispersed in the dispersion medium to obtain an initial suspension. Add the above primary suspension into a nano grinder, adjust the rotation speed to 1000 rpm, and wet grind for 120 min to obtain a nanoscale drug suspension. Add the prescribed amount of glycine and aspartame into the suspension, stir and mix until fully dissolved, and obtain a suspension for freeze-drying. After vacuum degassing the above suspension, inject it into the mold accurately. Freeze-dry the mold filled with the liquid medicine according to the freeze-drying curve: Pre-freeze at -40°C for 4 hours—vacuumize——15°C for 10 hours—0°C for 5 hours—15°C for 6 hours—30°C for 2 hours— Return to atmospheric pressure. Afte...
Embodiment 3
[0037] Recipe composition:
[0038]
[0039] Add hydroxypropyl cellulose and sodium lauryl sulfate into 150mL of pure water and stir to dissolve, add microcrystalline cellulose-sodium carboxymethyl cellulose slowly, and stir at high speed until uniformly dispersed. Slowly pour the celecoxib raw material into the dispersion medium and stir for 15 minutes, so that the celecoxib is fully wetted and dispersed in the dispersion medium to obtain an initial suspension. Add the above primary suspension into a nano grinder, adjust the rotation speed to 3500 rpm, and wet grind for 45 minutes to obtain a nanoscale drug suspension. Add the prescribed amount of sucrose and aspartame into the suspension, stir and mix until fully dissolved, and obtain a suspension for freeze-drying. After vacuum degassing the above suspension, inject it into the mold precisely. Freeze-dry the mold filled with the liquid medicine according to the freeze-drying curve: pre-freeze at -80°C for 2 hours—vacuu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com