A purification method of a Sakubaquat Sandan sodium intermediate
The technology of a sacubitril-valsartan and a purification method is applied in the field of purification of an intermediate of sacubitril-valsartan sodium, and can solve the problems of incomplete acid removal, low purity and yield, corrosiveness of instruments and equipment, and the like, Achieve the effect of overcoming corrosive problems and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
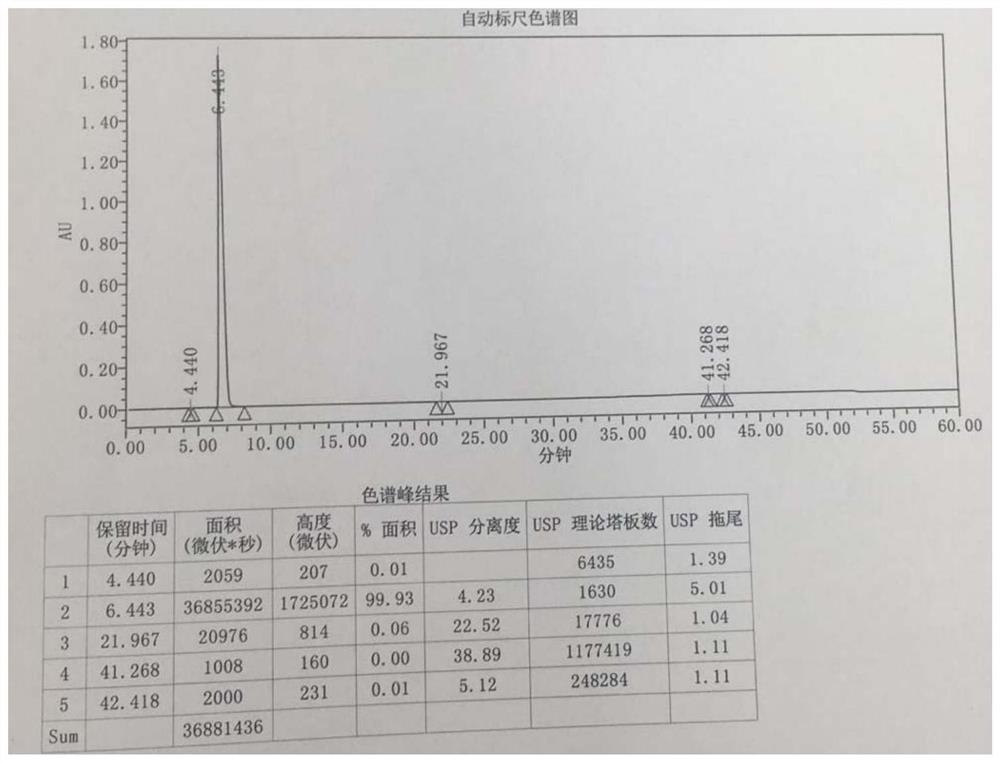
[0053] Example 1 Preparation of intermediate 1 ((2R,4S)-4-amino-5-biphenyl-4-yl-2-methylpentanoic acid ethyl ester hydrochloride)
[0054] The intermediate 1 was prepared and isolated by the following method:
[0055] S1. Dissolve 50 g of the starting material (2R,4S)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentanoic acid (1eq) in 380 mL of anhydrous In ethanol (50eq), cool down to 4°C, add 31.0g of thionyl chloride (2eq) dropwise at a rate of 1mL / min, keep the temperature below 10°C, raise the temperature to reflux after dropping, and stir at 75°C for 1.5h , get the reaction liquid;
[0056] S2. Cool the reaction solution obtained in step S1 to 50°C, concentrate under reduced pressure until no obvious liquid droplets flow out, then add 500mL of ethyl acetate, raise the temperature to 75°C, stir for 60min, and add 250mL of n-heptane after the system is clear, Cool down to below 5°C, stir and crystallize for 60 min, filter with suction, wash the filter cake w...
Embodiment 2
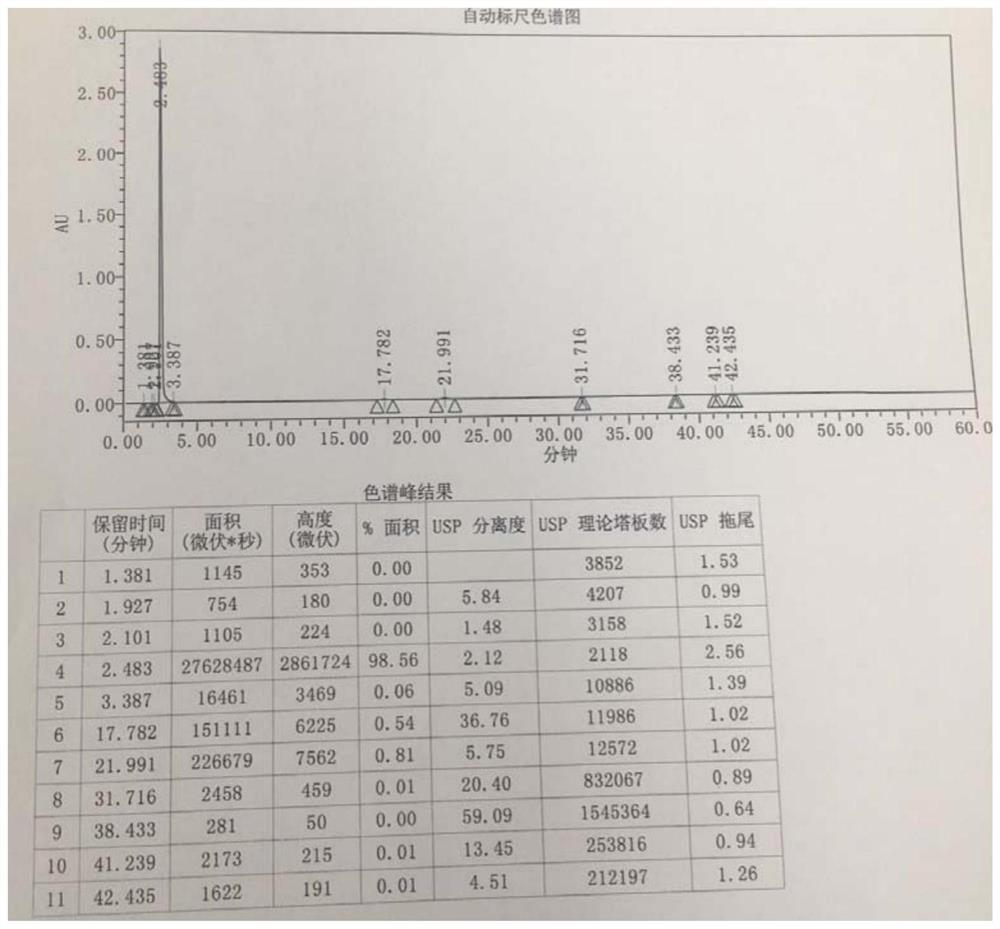
[0057] Example 2 Preparation of intermediate 1 ((2R,4S)-4-amino-5-biphenyl-4-yl-2-methylpentanoic acid ethyl ester hydrochloride)
[0058] The intermediate 1 was prepared and isolated by the following method:
[0059] S1. Dissolve 50 g of the starting material (2R,4S)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentanoic acid (1eq) in 380 mL of anhydrous In ethanol (50eq), lower the temperature to 4°C, add 31.0g of thionyl chloride (2eq) dropwise, keep the temperature below 10°C, raise the temperature to reflux after dropping, reflux at 75°C and stir for 1.5h to obtain a reaction solution;
[0060] S2. Cool the reaction solution obtained in step S1. to 50°C, concentrate under reduced pressure until no obvious liquid droplets flow out, add 500mL of ethyl acetate, raise the temperature to 75°C, stir for 60min, and add 375mL of n-heptane until the system is clear, Cool down to below 5°C, stir and crystallize for 60 min, filter with suction, wash the filter cake with...
Embodiment 3
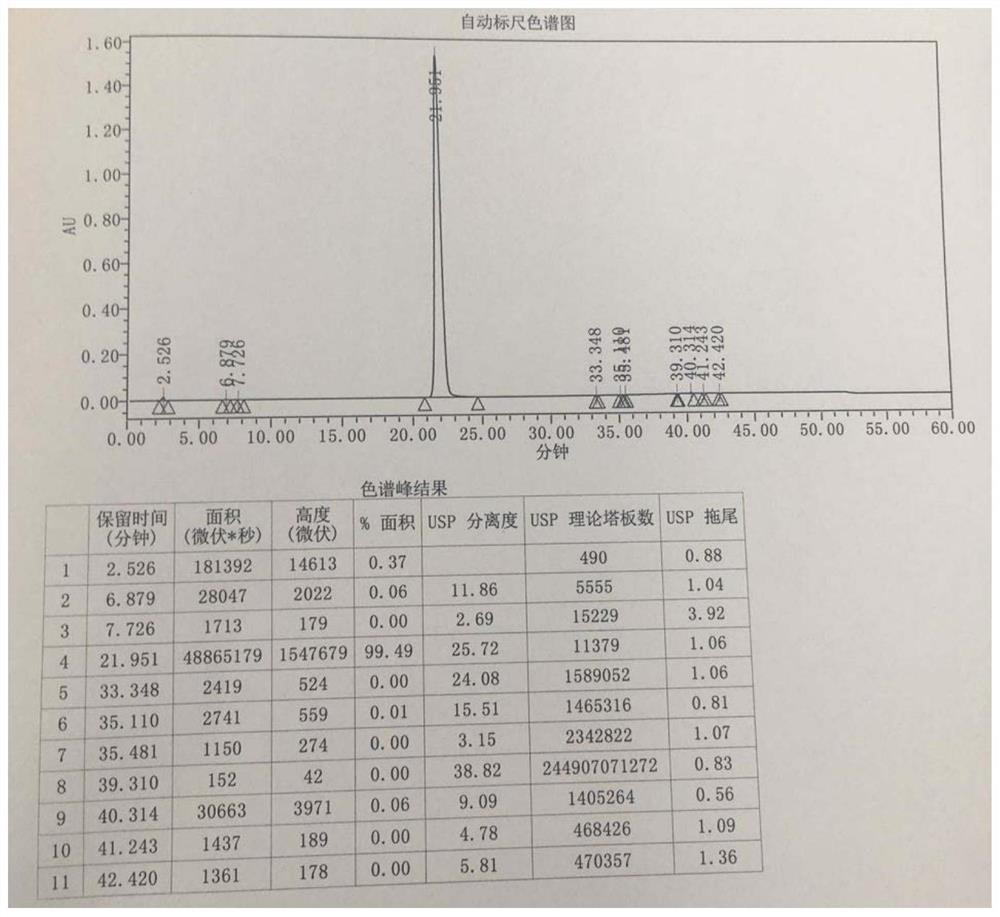
[0061] Example 3 Preparation of intermediate 1 ((2R,4S)-4-amino-5-biphenyl-4-yl-2-methylpentanoic acid ethyl ester hydrochloride)
[0062] The intermediate 1 was prepared and isolated by the following method:
[0063] S1. Dissolve 50 g of the starting material (2R,4S)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentanoic acid (1eq) in 380 mL of anhydrous In ethanol (50eq), lower the temperature to 4°C, add 31.0g of thionyl chloride (2eq) dropwise, keep the temperature below 10°C, raise the temperature to reflux after dropping, reflux at 75°C and stir for 1.5h to obtain a reaction solution;
[0064] S2. Cool the reaction solution obtained in step S1 to 50°C, concentrate under reduced pressure until no obvious liquid drops flow out, then add 500mL of ethyl acetate, raise the temperature to 75°C, stir for 60min, and add 500mL of n-heptane after the system is clear, Cool down to below 5°C, stir and crystallize for 60 min, filter with suction, wash the filter cake wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com