Novel lithium ion battery CsPbBr3/CNT perovskite composite material and preparation method thereof
A technology of nanocomposite materials and composite nanomaterials, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of negative electrode cycle stability and relative capacity density, and achieve controllable performance, high application value, The effect of the novel synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]1. Disperse 10 mg of carboxylated carbon nanotubes in 50 mL of DMF to make a 0.2 g / L dispersion, ultrasonically vibrate for 3 hours to make it evenly dispersed; then centrifuge at 6500 rpm for 10 minutes, take the supernatant, and set aside;
[0036] 2. Weigh CsBr and PbBr respectively according to the molar ratio of 1:1 2 0.1 mmol each, add 5 mL of the supernatant obtained in step 1, and ultrasonically dissolve it completely for 20 min; make it into 0.02 mol / L CsPbBr 3 / CNT precursor mixture; heated to 90°C with magnetic stirring to mix the mixture evenly;
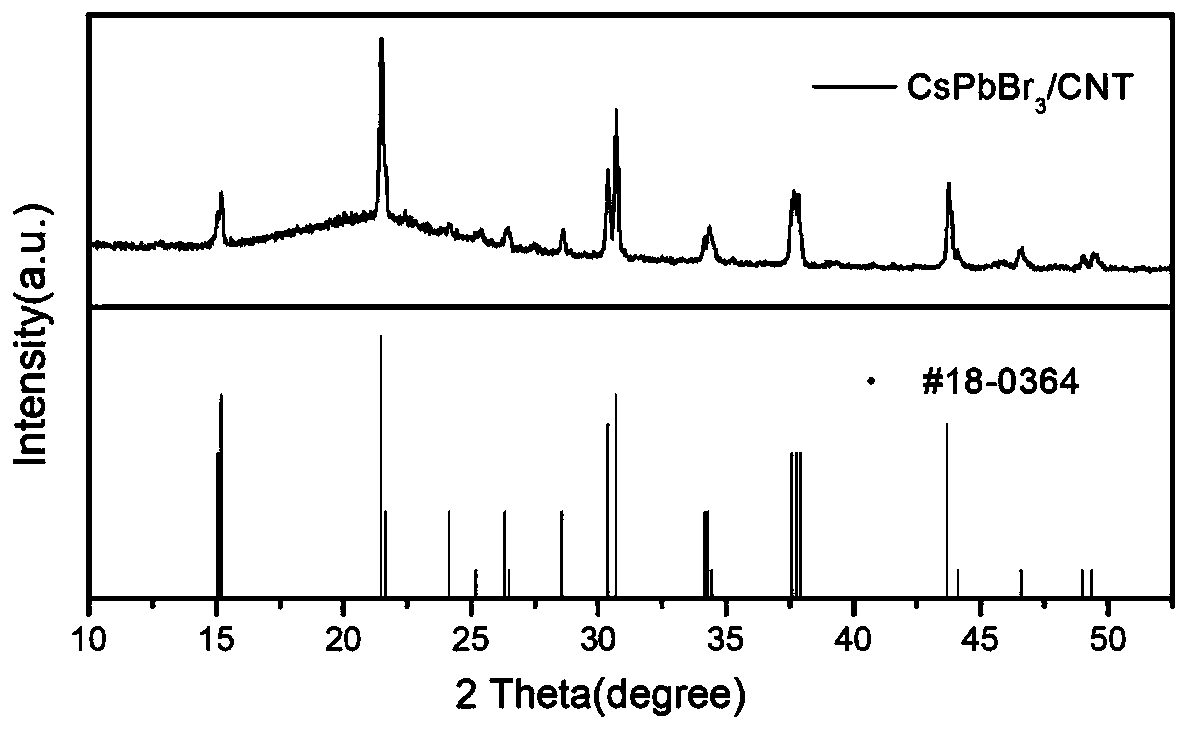
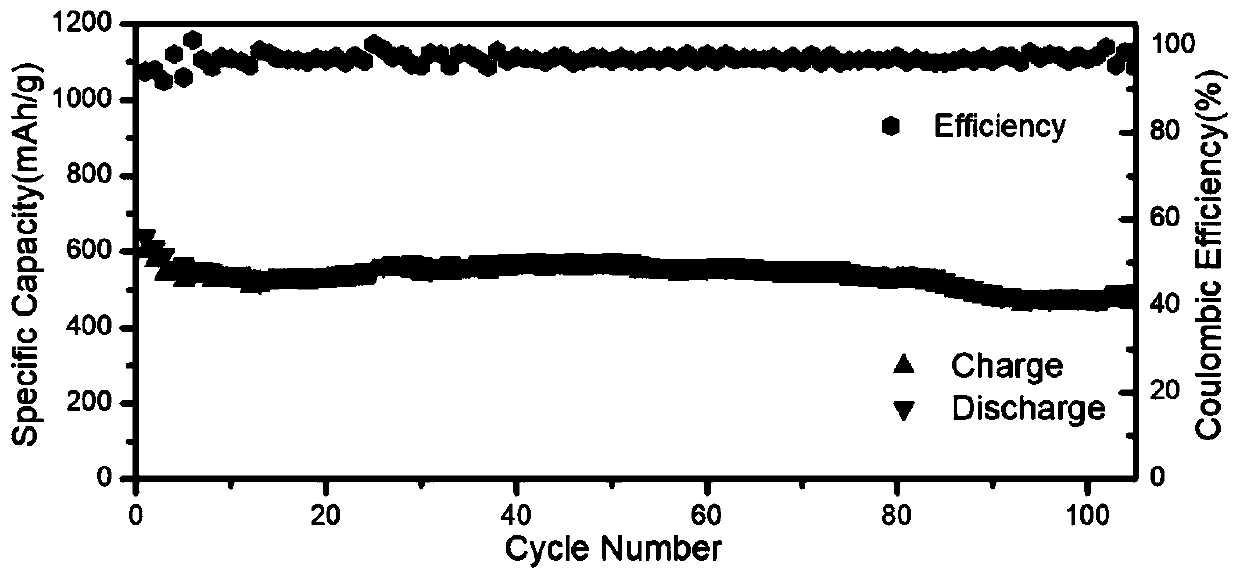
[0037] 3. According to the volume ratio of 1:10, take 5mL of the precursor mixture and add it to 50mL of toluene; after stirring for 1 hour at room temperature, wash with ethyl acetate for three times, and dry the precipitate in a vacuum oven at 60°C overnight to obtain CsPbBr 3 / CNT composite nanomaterials. The materials and electrochemical properties of the obtained products are shown in Table 1.
Embodiment 2
[0039] 1. Disperse 20mg of carboxylated carbon nanotubes in 40mL DMF to make a 0.5g / L dispersion, ultrasonically vibrate for 6h to make it evenly dispersed; then centrifuge at 6500rpm for 15min, take the supernatant, and set aside;
[0040] 2. Weigh CsBr and PbBr respectively according to the molar ratio of 1:1 2 0.1 mmol each, add 5 mL of the supernatant obtained in step 1, and ultrasonically dissolve the mixture completely for 20 min; make it into 0.02 mol / L CsPbBr 3 / CNT precursor mixture; heated to 95°C with magnetic stirring to mix the mixture evenly;
[0041] 3. According to the volume ratio of 1:40, take 0.5mL of the precursor mixture and add it to 20mL of toluene; after stirring at room temperature for 1 hour, centrifuge and wash with ethyl acetate three times, and dry the precipitate in a vacuum oven at 60°C overnight to obtain CsPbBr 3 / CNT composite nanomaterials. The materials and electrochemical properties of the obtained products are shown in Table 1.
Embodiment 3
[0043] 1. Disperse 5 mg of carboxylated carbon nanotubes in 50 mL of DMF to make a 0.1 g / L dispersion, ultrasonically vibrate for 3 hours to make it evenly dispersed; then centrifuge at 6500 rpm for 15 minutes, take the supernatant, and set aside;
[0044] 2. Weigh CsBr and PbBr respectively according to the molar ratio of 1:1 2 0.1 mmol each, add 10 mL of the supernatant obtained in step 1, and ultrasonically dissolve it completely for 10 min; make it into 0.01 mol / L CsPbBr 3 / CNT precursor mixture; heated to 70°C with magnetic stirring to mix the mixture evenly;
[0045] 3. According to the volume ratio of 1:4, take 10mL of the precursor mixture and add it to 40mL of toluene; after stirring for 1 hour at room temperature, wash with ethyl acetate for three times, and dry the precipitate in a vacuum oven at 60°C overnight to obtain CsPbBr 3 / CNT composite nanomaterials. The materials and electrochemical properties of the obtained products are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com