Perovskite type composite metal oxide oxygen carrier as well as preparation method and application thereof
A composite metal, perovskite-type technology, applied in the field of oxygen carriers, can solve the problems of poor structural stability of composite oxides, low reactivity of oxygen carriers, low selectivity of oxidation products, etc., and is conducive to industrial promotion and use, Low cost and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
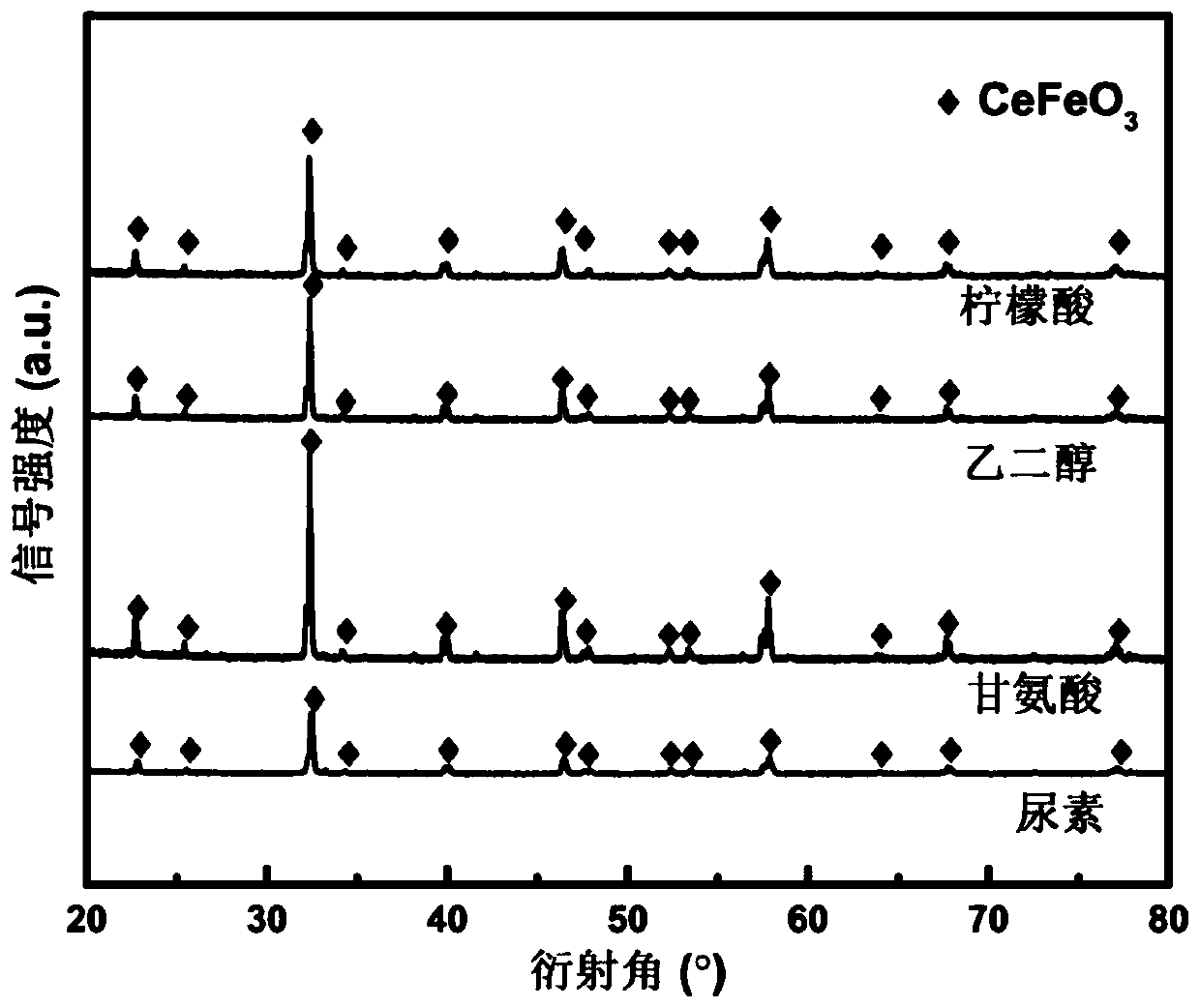
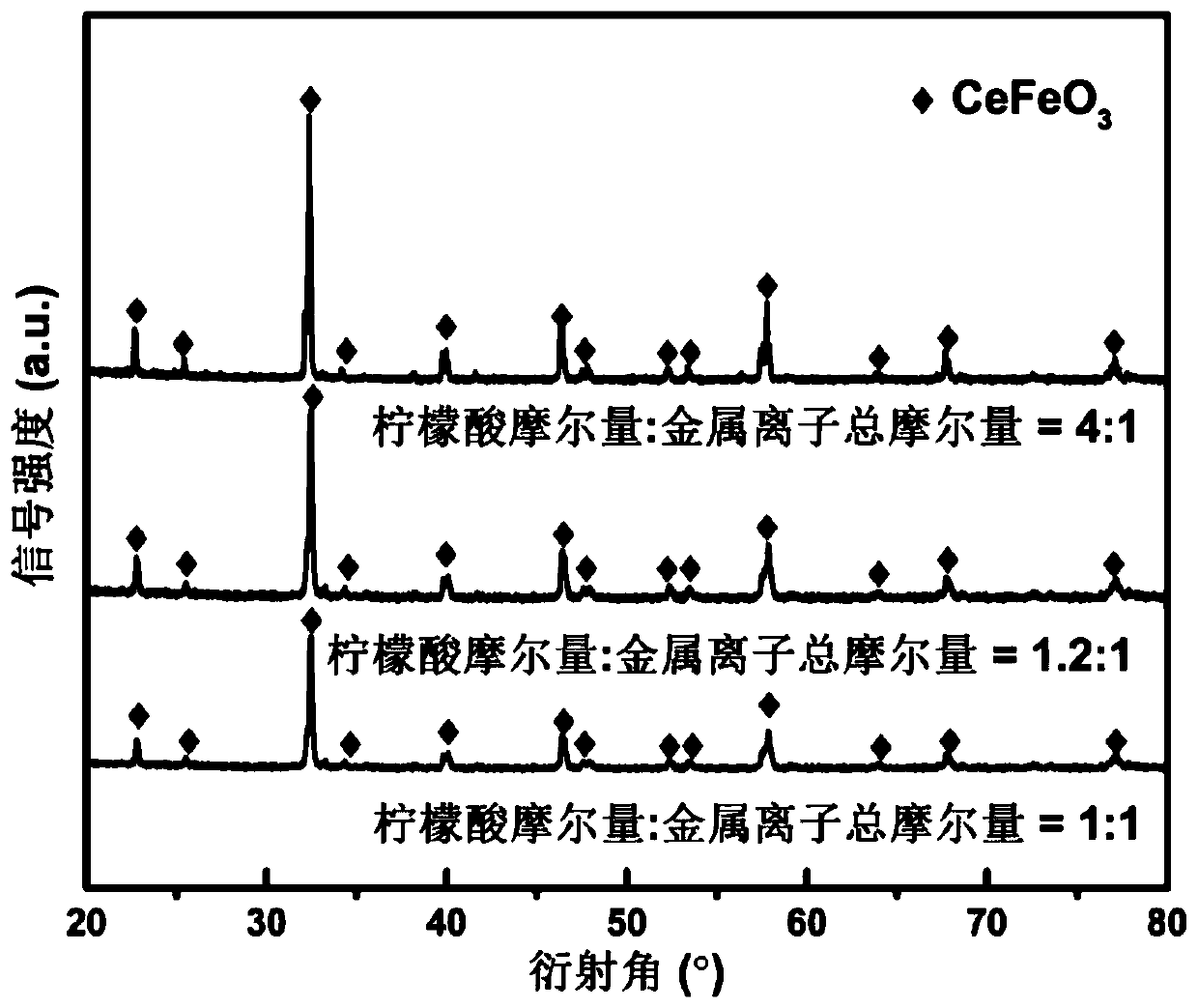
[0042] Step 1, weigh 4.3412g of cerium nitrate hexahydrate Ce (NO 3 ) 3 ·6H 2 O, 4.0399g of iron nitrate nonahydrate Fe (NO 3 ) 3 9H 2 O and complexing agent 4.6114g citric acid are dissolved in 50mL deionized water and are configured as a precursor solution, wherein the mol ratio of citric acid and the total amount of metal ions in the precursor solution is 1.2:1;
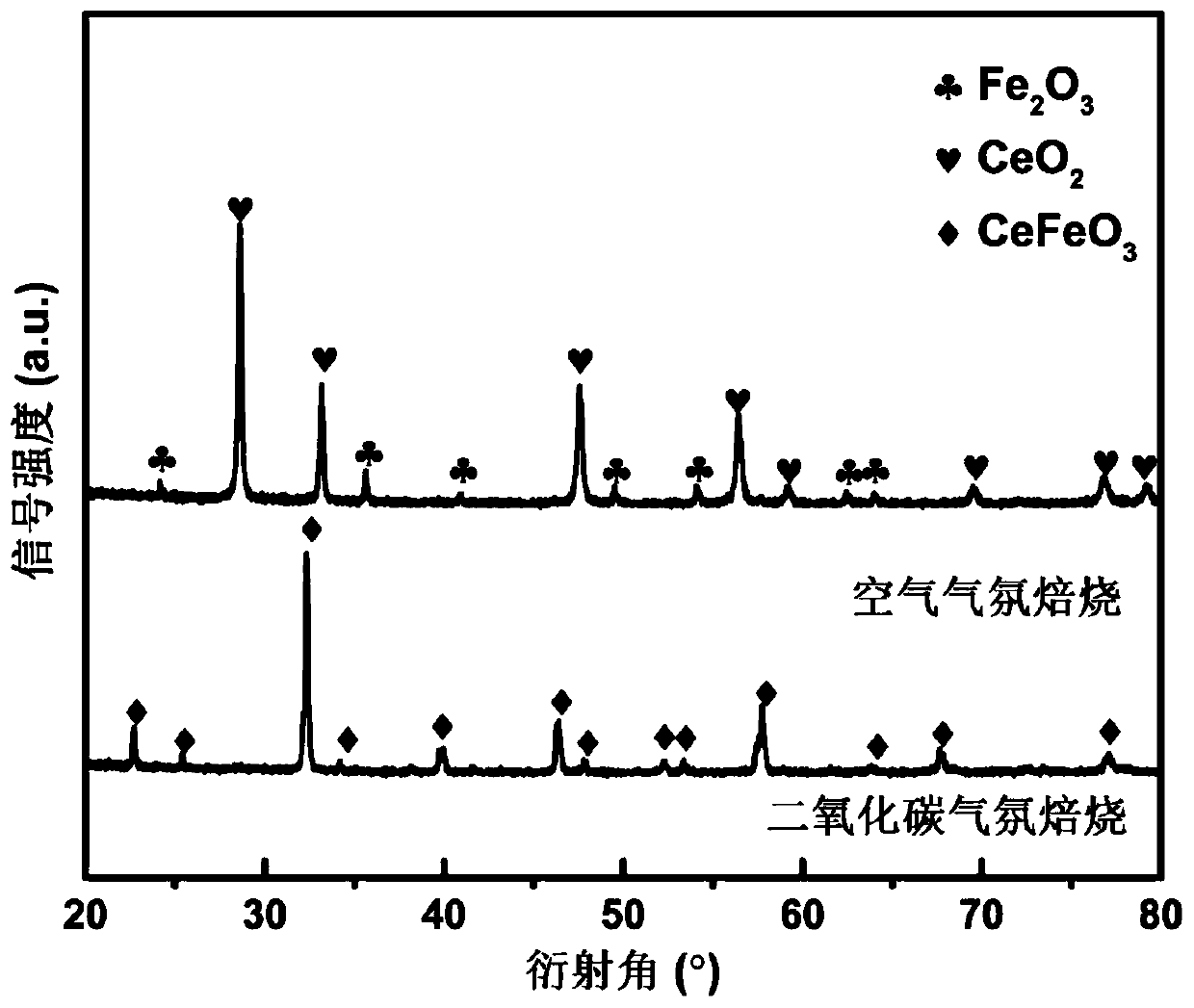
[0043] Step 2, the solution obtained in step 1 was evaporated to dryness at 90°C under stirring conditions until it became gelatinous, and the stirring rate was 600rpm; then it was aged in a constant temperature drying oven at 200°C for 24 hours, and the obtained precursor powder was dried at 1000°C in an air atmosphere Calcined for 6 hours to obtain the lanthanum cerium iron perovskite oxygen carrier, denoted as CeFeO 3 ;
[0044] Step 3, the CeFeO 3 The solid powder is compressed into tablets, sieved, and the granular oxygen carrier with a size of 20-40 mesh is taken.
Embodiment 2
[0046] Step 1, weigh 4.3412g of cerium nitrate hexahydrate Ce (NO 3 ) 3 ·6H 2 O, 4.0399g of iron nitrate nonahydrate Fe (NO 3 ) 3 9H 2 O and complexing agent 4.6114g citric acid are dissolved in 50mL deionized water and are configured as a precursor solution, wherein the mol ratio of citric acid and the total amount of metal ions in the precursor solution is 1.2:1;
[0047] Step 2, the solution obtained in step 1 was evaporated to dryness at 90°C under stirring conditions until it became gelatinous, and the stirring rate was 600rpm; then it was aged in a constant temperature drying oven at 200°C for 24 hours, and the obtained precursor powder was dried at 1000°C in a carbon dioxide atmosphere Calcined for 6 hours to obtain the lanthanum cerium iron perovskite oxygen carrier, denoted as CeFeO 3 ;
[0048] Step 3, the CeFeO 3 The solid powder is compressed into tablets, sieved, and the granular oxygen carrier with a size of 20-40 mesh is taken.
Embodiment 3
[0050] Prepared with the method of Example 2, the only difference is that the complexing agent in step (1) uses 1.4900g ethylene glycol, and the molar ratio of ethylene glycol to the total amount of metal ions in the precursor solution is 1.2:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com