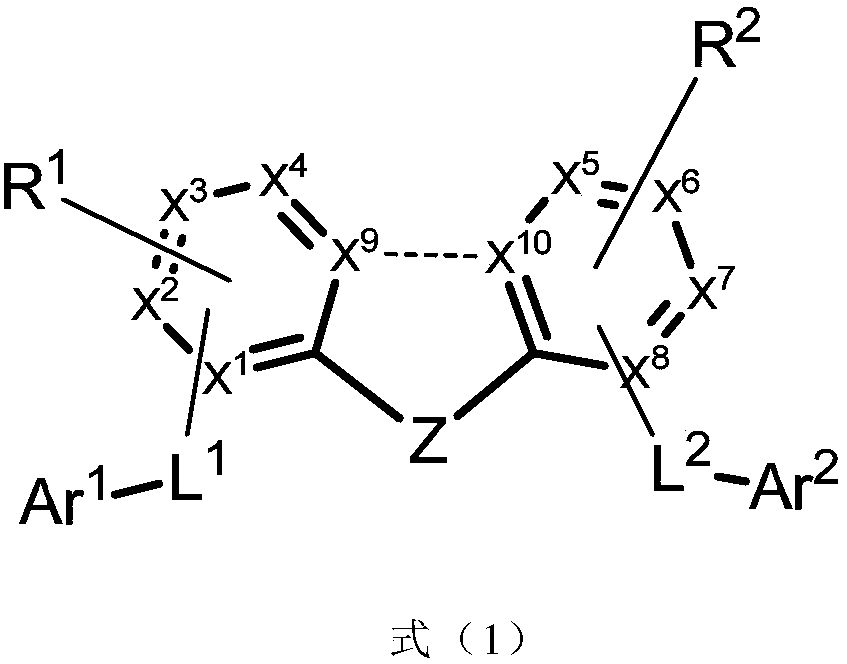
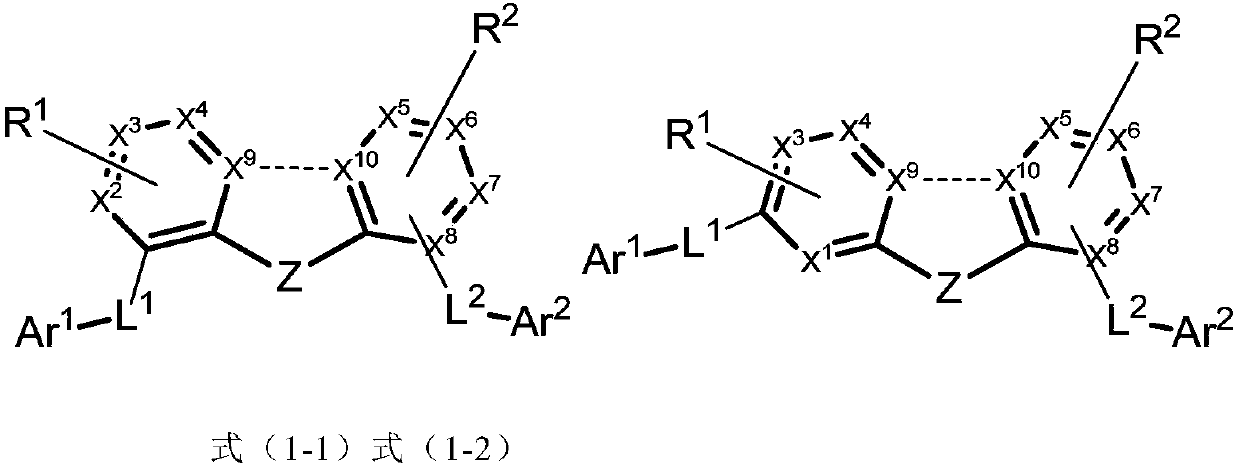
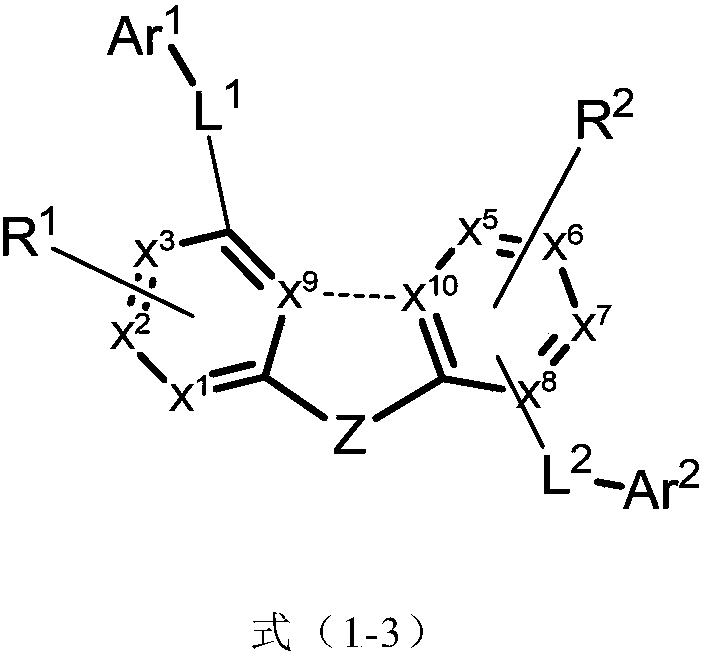
Imidazole compound and applications thereof
A general formula compound, unsubstituted technology, applied in the direction of organic chemistry, chemical instruments and methods, electrical components, etc., can solve the problems of unreported applications, stability needs to be improved, low triplet energy level, etc., to reduce conjugation degree, improve device efficiency and life, and reduce the effect of implantation energy barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0053] Synthesis Example 1: Synthesis of M1
[0054]
[0055] Synthesis of intermediate M1-1:
[0056] 12.2g (100mmol) of o-hydroxybenzaldehyde and 18.4g (100mmol) of o-bromobenzaldehyde were added to a newly dried 1000mL two-necked bottle, and 20.7g (150mmol) of anhydrous potassium carbonate and 600mL of dried potassium carbonate were added under nitrogen protection. N,N-dimethylformamide (DMF), stirred at room temperature for 5 hours, then heated to 130 degrees Celsius to continue the reaction for 10 hours. After the reaction was completed, it was lowered to room temperature, and the solvent in the reaction system was distilled off under reduced pressure. The crude product was dissolved in 500 mL of dichloromethane and washed with a large amount of water. The organic phase was dried over anhydrous sodium sulfate and then concentrated. Column chromatography was performed with dichloromethane:petroleum ether=1:1 as the eluent to obtain 20 g of off-white solid with a yield ...
Synthetic example 2
[0059] Synthesis Example 2: Synthesis of M2
[0060]
[0061] Synthesis of intermediate M2-1:
[0062] 12.2g (100mmol) o-hydroxybenzaldehyde and 32.1g (100mmol) 9-(2-bromophenyl)-9H-carbazole were added to a freshly dried 1000mL two-necked bottle, and 20.7g ( 150 mmol) of anhydrous potassium carbonate and 600 mL of dry N,N-dimethylformamide (DMF), stirred at room temperature for 5 hours, then heated to 130 degrees Celsius to continue the reaction for 10 hours. After the reaction was completed, it was lowered to room temperature, and the solvent in the reaction system was distilled off under reduced pressure. The crude product was dissolved in 500 mL of dichloromethane and washed with a large amount of water. The organic phase was dried over anhydrous sodium sulfate and then concentrated. Column chromatography was performed with dichloromethane:petroleum ether=1:3 as the eluent to obtain 35 g of off-white solid with a yield of 96%. The molecular mass determined by mass spe...
Synthetic example 3
[0065] Synthesis Example 3: Synthesis of M46
[0066]
[0067] Synthesis of Intermediate M46-1:
[0068] Add 32.4g (100mmol) of 4,6-dibromodibenzothiophene and 112.8g (300mmol) of 2-formylphenylboronic acid into a freshly dried 3000mL two-necked bottle, and add 62.1g (450mmol) under nitrogen protection Anhydrous potassium carbonate, 6.9 g (6 mmol) palladium tetrakistriphenylphosphine and 225 mL water and 1500 mL 1,4-dioxane. After the reaction was completed, it was lowered to room temperature, and the solvent in the reaction system was distilled off under reduced pressure. The crude product was dissolved in 500 mL of dichloromethane and washed with a large amount of water. The organic phase was dried with anhydrous sodium sulfate and then concentrated. Column chromatography was performed with dichloromethane:petroleum ether=1:1 as the eluent to obtain 30 g of off-white solid with a yield of 80%. The molecular mass determined by mass spectrometry is: 376.05 (calculated val...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com