Method for preparing uridylic acid by enzyme method
A technology for uridylic acid and uridine, which is applied in the field of enzymatic preparation of uridylic acid, can solve the problems of difficult product separation, poor production safety, explosiveness and the like, and achieves the effects of reduced cost, low cost, and safe and reliable preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
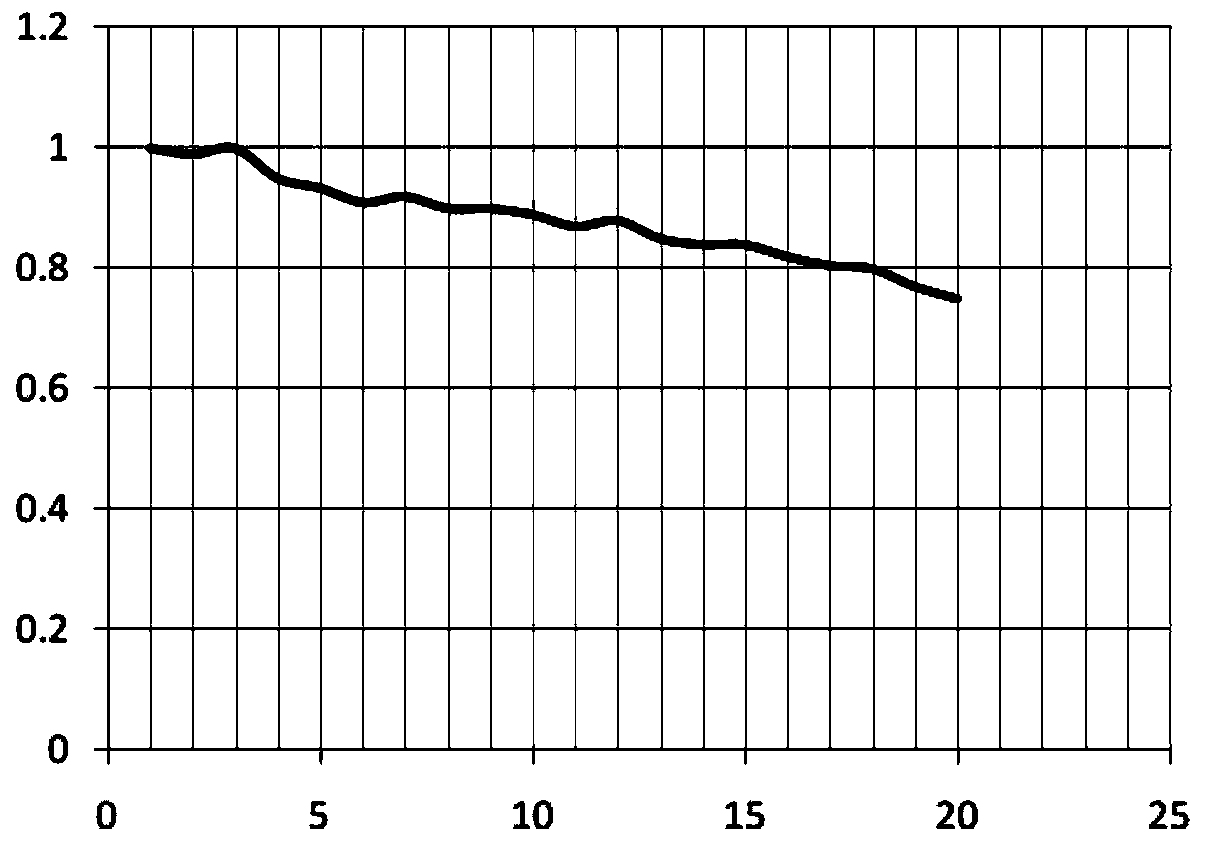
Image
Examples
Embodiment 1
[0029] Embodiment 1, preparation UMP production enzyme
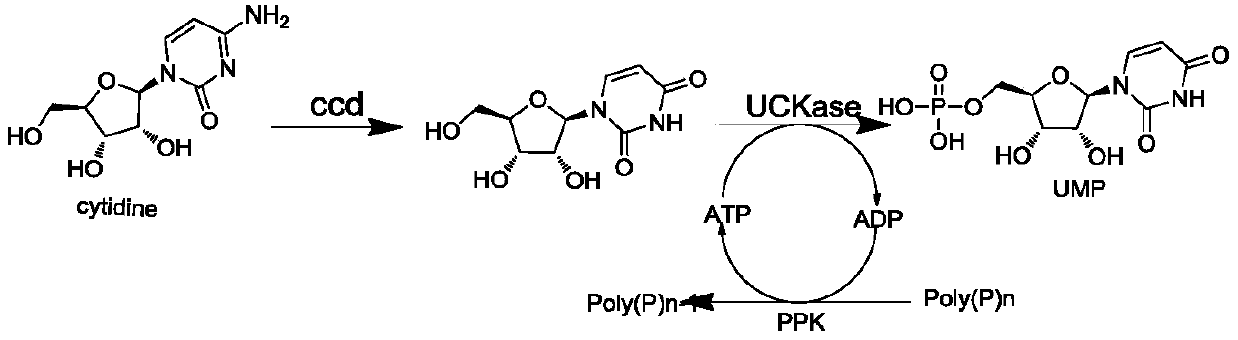
[0030] According to the sequences of the three enzyme genes, three pairs of amplification primers were designed. Genomic DNA of Escherichia coli strains was extracted, using it as a template, PCR amplified cytidine deaminase (ccd) and polyphosphate kinase (PPK1) gene fragments, and respectively ligated them into the pET28a vector ( purchased from Novagene Company); extract Lactobacillus bulgaricus (Lactobacillus bulgaricus) genomic DNA, and use it as a template to amplify the uridine-cytidine kinase (UCK) gene fragment by PCR, and connect it to the pET 28a vector (purchased from Novagene Company ). After the three gene fragments were successfully connected and sequenced correctly, they were respectively transferred into E.coli BL21 (DE3) strains (Shanghai Weidi Biotechnology Co., Ltd.).
[0031] 其中,胞苷脱氨酶(cytidine deaminase,ccd)的序列为ATGCATCCACGTTTTCAAACCGCTTTTGCCCAACTTGCGGATAACTTGCAATCTGCACTGGAACCTATTCTGGCAGACAAGTACTTCCC...
Embodiment 2
[0038] Embodiment 2, use free enzyme to prepare UMP
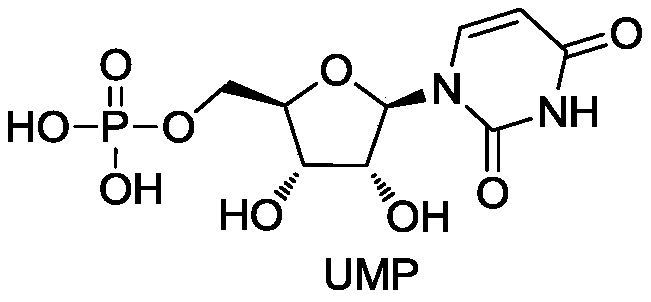
[0039] Substrate cytidine 100g, sodium hexametaphosphate 400g, ATP5g, MgCl 2 ·6H 2 O 50g, added to 5L pH7.0 phosphate buffer solution, stirred evenly, and adjusted the pH to 7.0. Add cytidine deaminase: 1000U / L, polyphosphate kinase: 600U / L, uridine-cytidine kinase: 1200U / L to the reaction liquid, control the pH during the reaction and keep it at 7.0, and the reaction temperature is 30-35°C. After reacting for 10 hours, the amount of UMP produced in the reaction supernatant liquid detected by HPLC was 23.5 g / L, the purity was 70%, and the conversion rate of cytidine was 85%.
[0040] HPLC detection conditions: Octadecylsilane bonded silica gel is used as filler, mobile phase A is tetrabutylammonium hydrogen phosphate, B phase is acetonitrile, detection wavelength is 260nm, flow rate is 1ml / min, column temperature is 30°C, elution The program is shown in Table 1.
[0041] Table 1 Elution program
[0042] time ...
Embodiment 3
[0044] Embodiment 3, use immobilized cell to prepare UMP
[0045] Substrate cytidine 80g, sodium hexametaphosphate 300g, ATP5g, MgCl 2 ·6H 2 O60g, add 5L pH7.0 phosphate buffer solution, stir evenly, adjust pH to 7.0. Add the immobilized cells of each enzyme in the reaction solution, wherein the activities are respectively cytidine deaminase: 2000U / L, polyphosphate kinase: 800U / L, uridine-cytidine kinase: 1500U / L, and the pH is controlled during the reaction Keep it at 7.0, and the reaction temperature is 30-35°C. After reacting for 15 hours, the amount of UMP produced in the reaction supernatant was detected by HPLC to be 16.4 g / L, the conversion rate of cytidine was 75%, and the purity was 53%. HPLC detection condition is the same as embodiment 2.
[0046]The supernatant collected by filtration was subjected to ion-exchange chromatography on a macroporous strongly basic anion-exchange resin, concentrated, crystallized, and dried to obtain 82 g of finished product UMP wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com