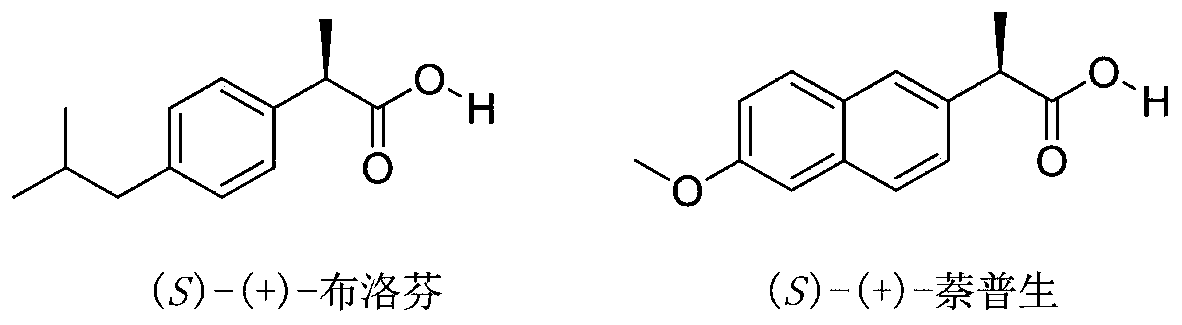
Synthetic method of chiral 2-aryl propionate
A technology of aryl propionate and aryl acrylate, which is applied in the field of enantioselective synthesis of 2-aryl propionate, can solve the problems of high pressure, high transition metal price, limited industrial application, etc. cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
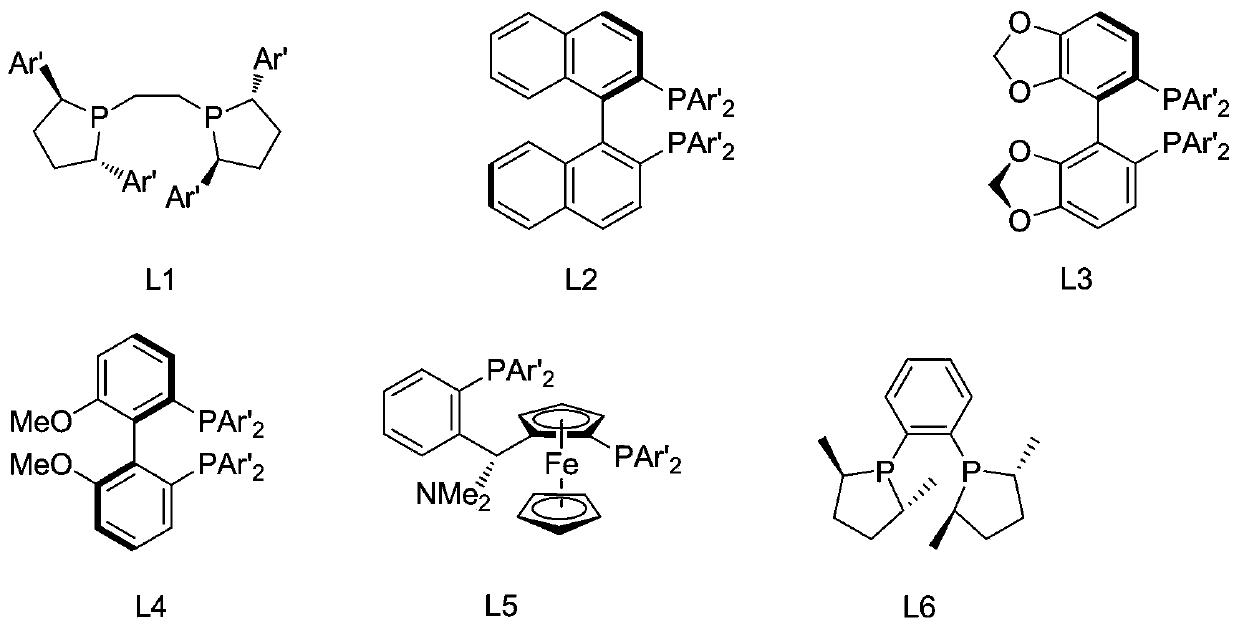
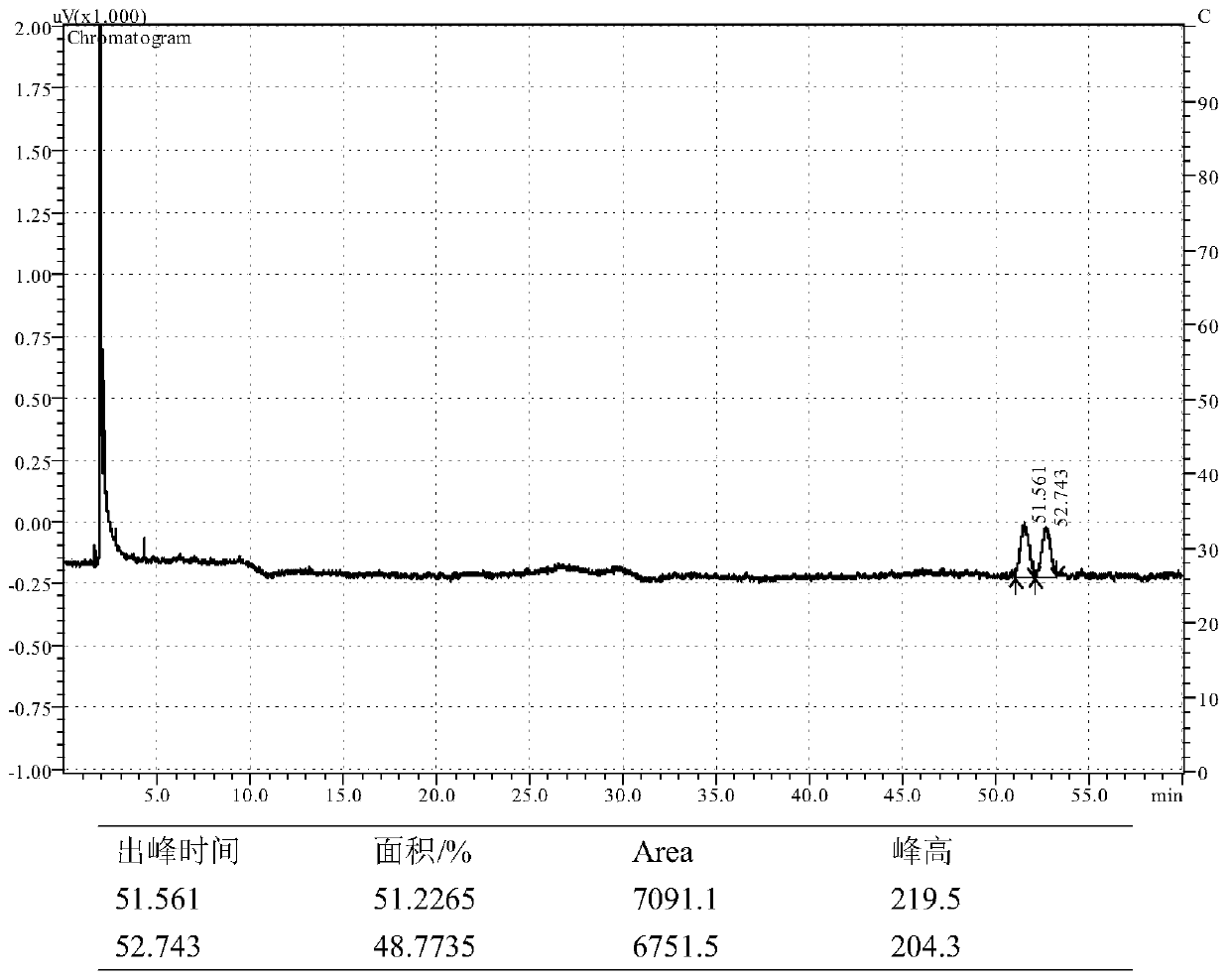
[0040] Add Cu(OAc) to nitrogen-purged, dry reaction flask 2 ·H 2 O (2.0mg), (R,R)-L1(Ar'=Ph) (2.0mg) and toluene (2.0mL) were stirred at room temperature to a blue solution. Add poly(methylhydrogensiloxane) (120 μL) and n-butanol (118 μL) to the above mixture, stir for 5 min, then add methyl 2-phenylacrylate (0.065 g), and stir for another 2 h. Afterwards, saturated NH 4 F solution (4.0mL), continued to stir for 60min, separated, the aqueous phase was extracted with ethyl acetate (3×5.0mL), the combined organic phase was washed with saturated NaCl solution, dried over anhydrous sodium sulfate, concentrated, and separated by column to obtain 0.046 g of methyl 2-phenylpropionate, the yield is 70%; the enantiomeric composition is analyzed by chiral GC, ee is 8%, and the dominant configuration is (S)-configuration. 1 H NMR (500MHz, CDCl 3 )δ7.32–7.20(m,5H),3.66(q,J=7.1Hz,1H),3.59(s,3H),1.43(d,J=7.2Hz,3H). 13 C NMR (126MHz, CDCl 3 )δ175.13, 140.67, 128.77, 127.59, 127.26, 52....
Embodiment 2
[0042] Add Cu(OAc) to a nitrogen-purged, dry reaction flask 2 ·H 2 O (2.0 mg), (R)-L2 (Ar'=Ph) (3.0 mg) and tetrahydrofuran (2.0 mL) were stirred at room temperature to a blue solution. Poly(methylhydrogensiloxane) (180 μL) and tert-butanol (140 μL) were added to the above system, after stirring, methyl 2-phenylacrylate (162 mg) was added, and the reaction was stirred for 2 h. Add saturated NH to the reaction mixture 4 Cl solution (4.0mL), continued to stir for 20min, separated, the aqueous phase was extracted with ethyl acetate (3×5.0mL), the combined organic phase was washed with NaCl saturated solution, dried over anhydrous sodium sulfate, concentrated, and column separated to obtain 101 mg of colorless liquid methyl 2-phenylpropionate, yield 62%; analyzed by chiral GC, the ee value was 19%, and the dominant configuration was (S)-configuration.
Embodiment 3
[0044] Add Cu(OAc) to a nitrogen-purged, dry reaction flask 2 ·H 2 O (2.0 mg), (S)-L3 (Ar'=Ph) (6.1 mg), toluene (2.0 mL), stirred at room temperature to a blue solution. Add Ph to the above liquid 2 SiH 2 (184μL), neopentylbutanol (132mg), and after stirring for 5min, 2-phenylmethylacrylate (152mg) was added, and the stirring reaction was continued for 2h. Add saturated NH to the reaction mixture 4 Cl solution (4.0mL), continued to stir for 20min, separated, the aqueous phase was extracted with ethyl acetate (3×5.0mL), the combined organic phase was washed with NaCl saturated solution, dried over anhydrous sodium sulfate, concentrated, and column separated to obtain Methyl 2-phenylpropanoate (111 mg, 72% yield), GC chiral analysis, ee 22%), the (S)-configuration was predominant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com