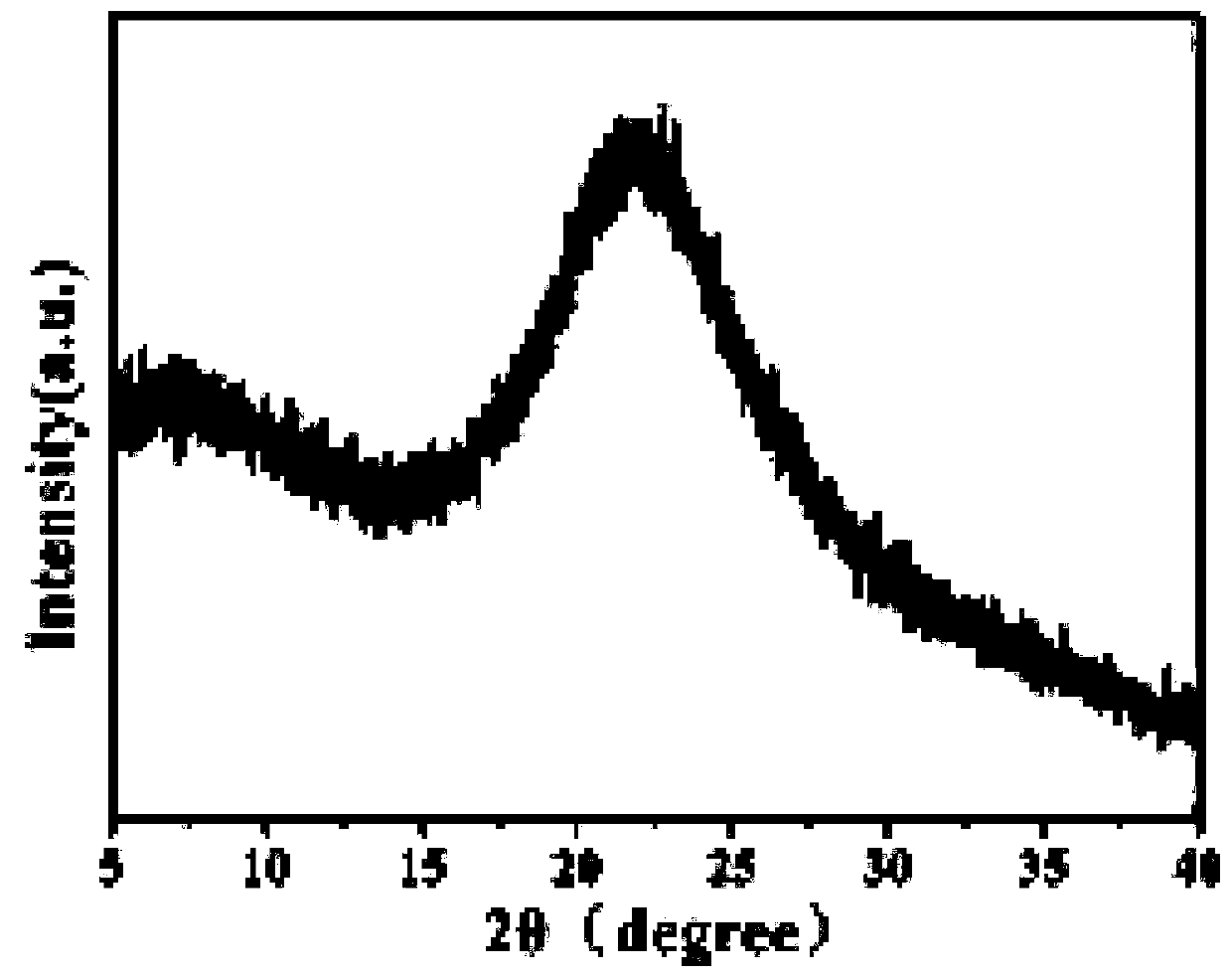
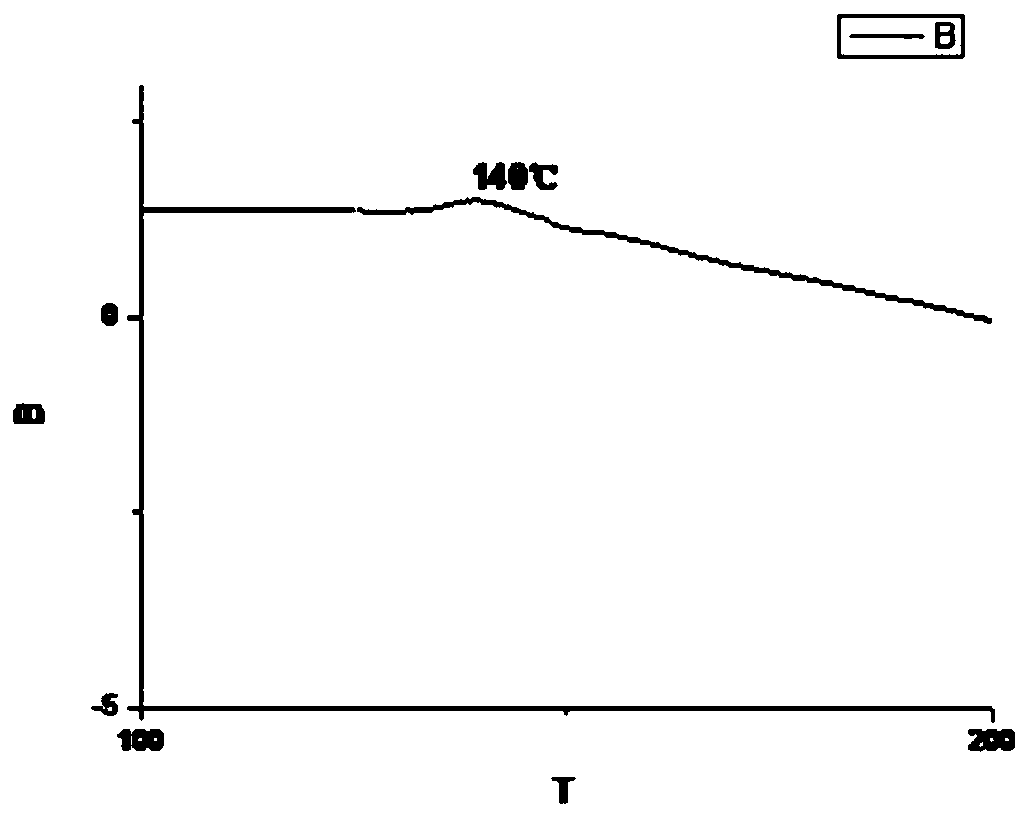
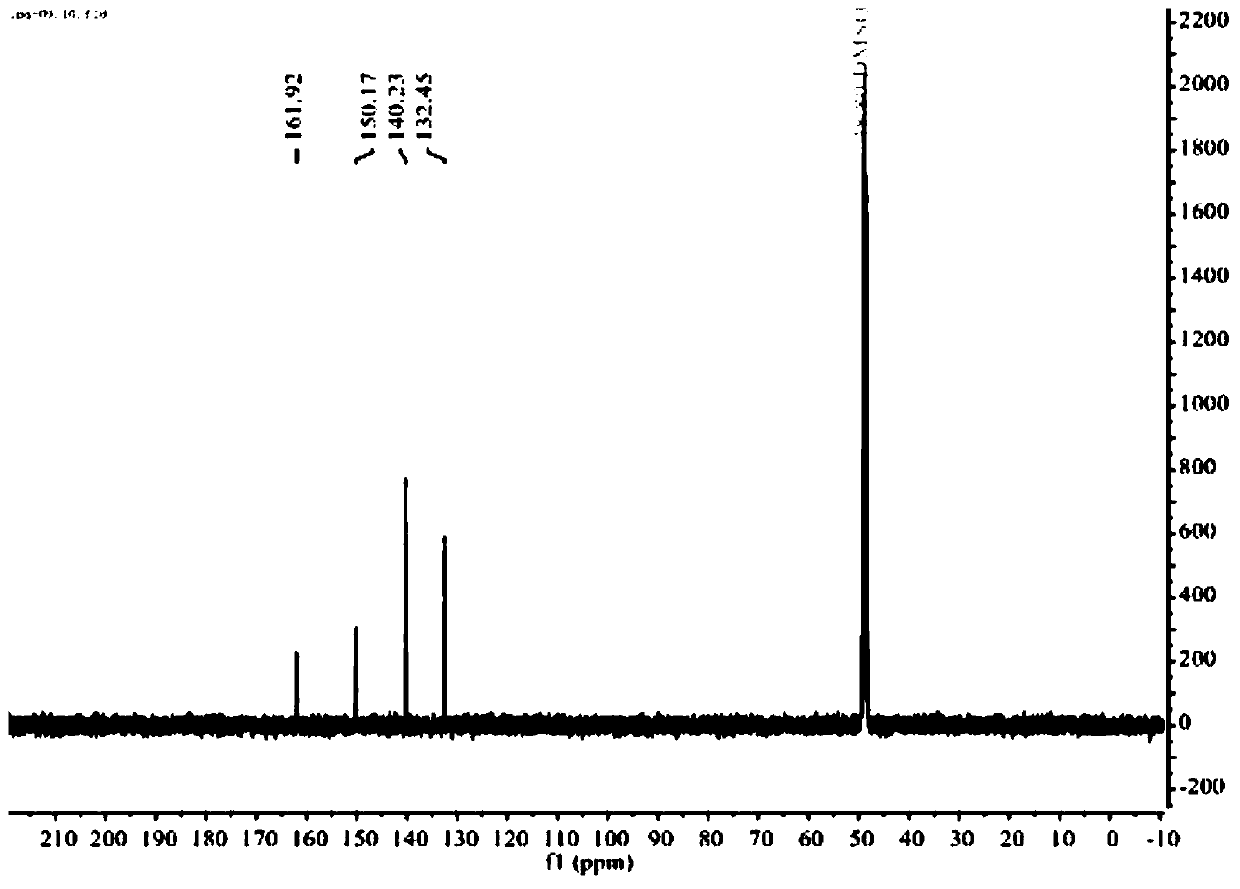
Synthesis method of novel bisphenol S derivative type polysulfate
A technology of polysulfate ester and synthesis method, which is applied in the field of polymer materials, can solve the problems of low dielectric loss, incomplete reaction of bisphenol S sulfonyl fluoride monomer, etc., and achieve low orientation polarization temperature and simple and stable synthesis process , The effect of simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A kind of synthetic method of novel bisphenol S derivative type polysulfate, comprises the following steps:
[0038] 1. Preparation of 4,4-disulfonyl fluoride diphenyl sulfone
[0039] (1) First put 1000g of bisphenol S monomer in a 50L autoclave, with a purity of 99.5% or more; put in 20L of DCM, stir in the autoclave, and stir at room temperature; add 971g of triethylamine, the temperature is 25°C, and stir for 30min Finally, inject 960g of sulfuryl fluoride gas, and the reaction time is 13h.
[0040] (2) Rotate the mixture obtained in (1) under reduced pressure in a 35°C water bath, spin out the solvent DCM and recover it, add 490ml of methanol solution and 1225ml of water to the remaining system, heat to boil at 90°C and reflux, mechanically After stirring, the solid was filtered out to obtain the target product, and 1324 g of white powder was obtained.
[0041](3) The aqueous methanol solution obtained in (2) was distilled under reduced pressure in a water bath a...
Embodiment 2
[0046] 1. Preparation of 4,4-disulfonyl fluoride diphenyl sulfone
[0047] Preparation method is identical with embodiment 1
[0048] Two, the synthesis of bisphenol S derivative type polysulfate
[0049] (1) Weigh 1000g of bisphenol S, 1672.5g of 4,4-disulfonyl fluoride diphenyl sulfone, 493g of potassium hydroxide, and 16.13L of nitrobenzene solution, and add them to the polymerization kettle at one time and stir, and the temperature rises from room temperature to to 50°C-150°C. The heating time is 2h, 6h, and 2h. After the reaction is completed, the material is discharged under pressure with nitrogen and settled in the ethanol solution.
[0050] (2) Put the polymer obtained in (1) into a 10L autoclave, add 5L of purified water, boil at 120°C for 5 hours, repeat once, after drying, use 6L methanol alcohol solution to boil once at 100°C, and dry , to obtain bisphenol S derivative polysulfate.
Embodiment 3
[0052] 1. Preparation of 4,4-disulfonyl fluoride diphenyl sulfone
[0053] Preparation method is identical with embodiment 1
[0054] Two, the synthesis of bisphenol S derivative type polysulfate
[0055] (1) Weigh 1000g of bisphenol S, 1689g of 4,4-disulfonyl fluoride diphenyl sulfone, 493g of potassium hydroxide, and 16.13L of nitrobenzene solution, add them into the polymerization kettle at one time and stir, and the temperature rises from room temperature to 50 ℃-150℃. The heating time is 2h, 6h, and 2h. After the reaction is completed, the material is discharged under pressure with nitrogen and settled in the ethanol solution.
[0056] (2) Put the polymer obtained in (1) into a 10L autoclave, add 5L of purified water, boil at 120°C for 5 hours, repeat once, after drying, use 6L methanol alcohol solution to boil once at 100°C, and dry Dry to obtain bisphenol S derivative type polysulfate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com