Nuclide marked trastuzumab monoclonal antibody as well as preparation method and application thereof
A trastuzumab and labeling technology, applied in the field of nuclear medicine, can solve problems such as drug resistance and recurrence, and achieve stable properties, low systemic background, and good imaging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 New nuclide 124 Preparation of I-labeled Trastuzumab
[0027] 124 I-Trastuzumab is prepared by NBS reaction: to 0.7Ml (45KBq / μL) Na 124 Add 0.5ml 0.1M PB (pH7.2) buffer, 0.1mL (10mg / mL) Trastuzumab monoclonal antibody solution (prepared with water for monoclonal antibody solution) and 12μg NBS to the I solution in sequence. React at 37°C for 60 seconds, and add 10% HSA to terminate the reaction. After the reaction solution is purified by PD-10 column, the target product is obtained 124 I-Trastuzumab.
[0028] The labeling rate is greater than 95%. After purification with a PD-10 pre-packed gel column, the radiochemical purity of the target compound is greater than 99%. Before use, the PD-10 column needs to be equilibrated with 0.01M pH 7.4PBS, adding 5 mL each time, draining by gravity flow rate, and repeating 5 times. Then use 0.01M pH 7.4PBS to purify the target compound.
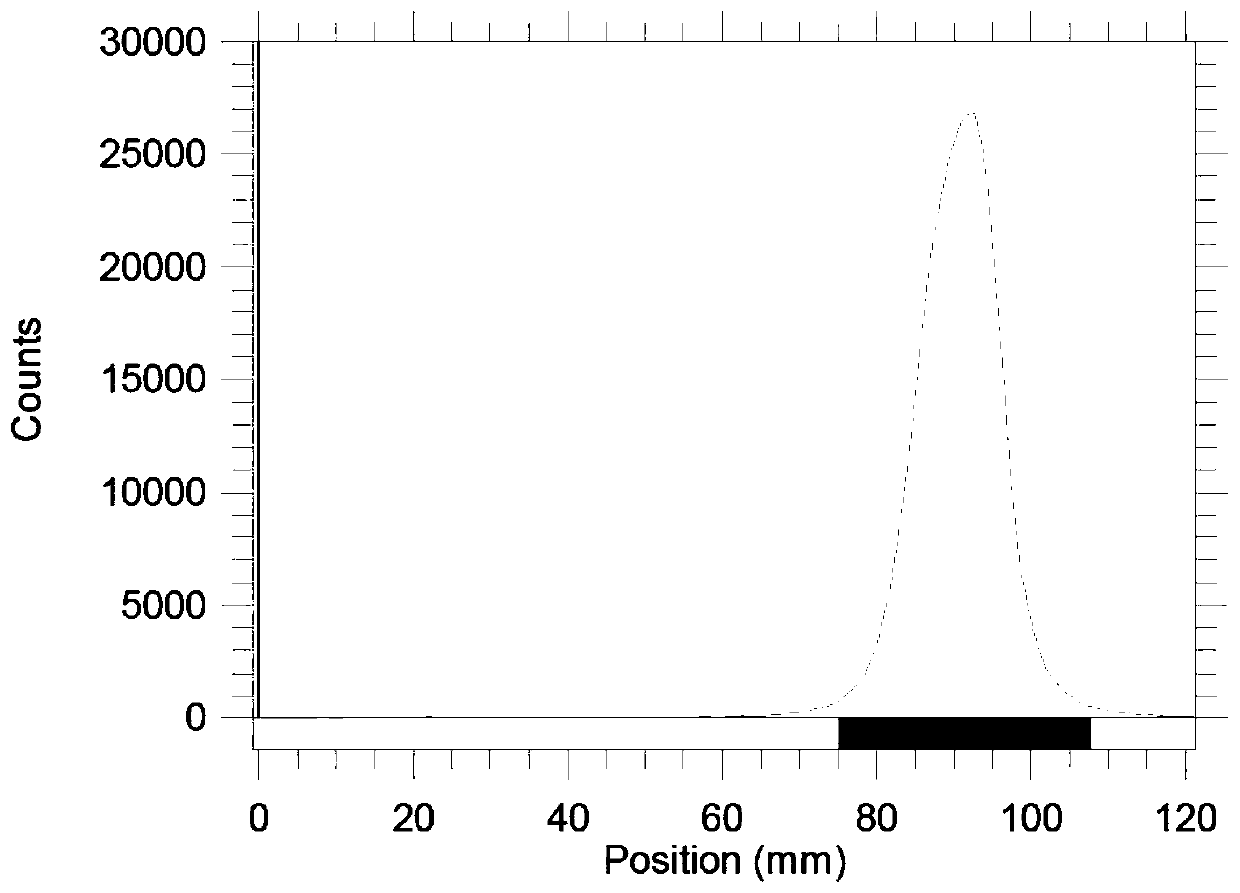
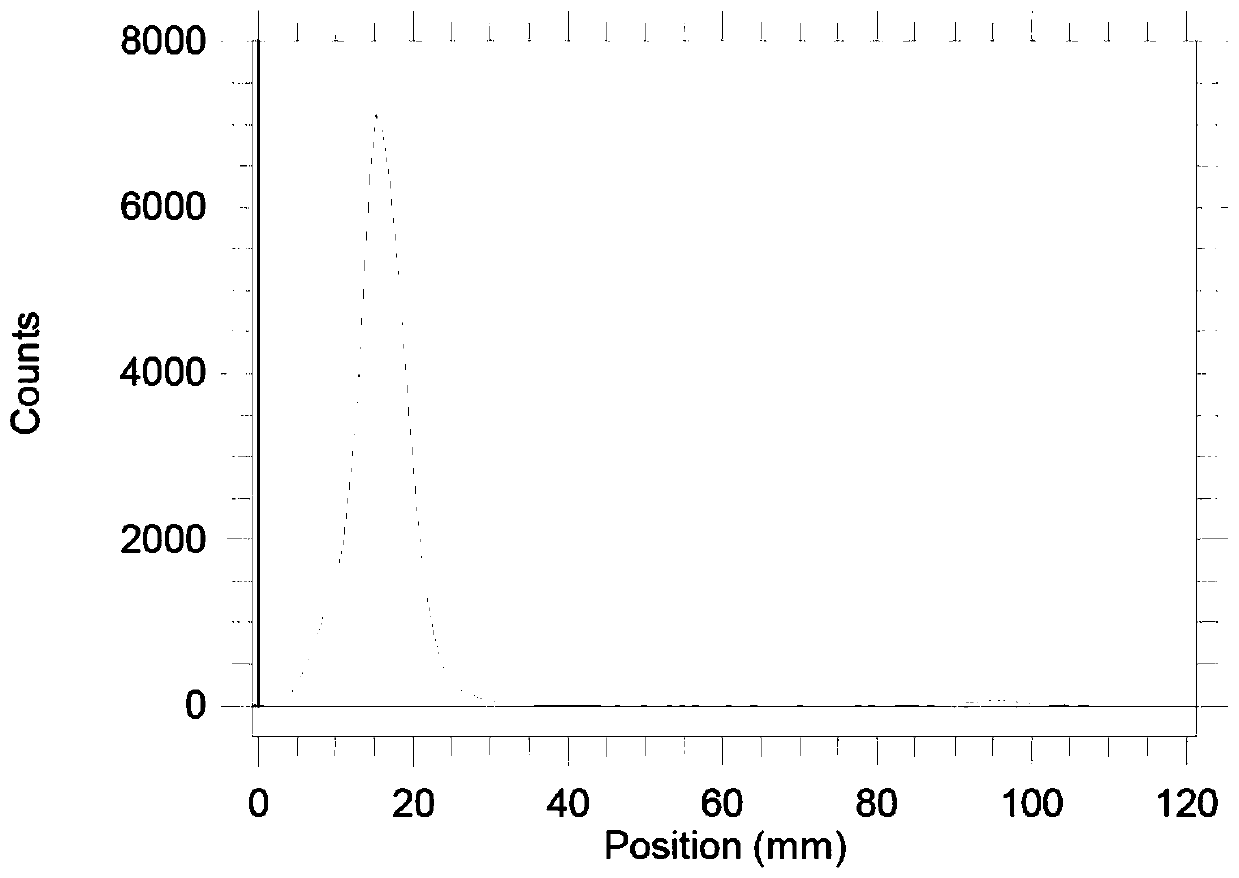
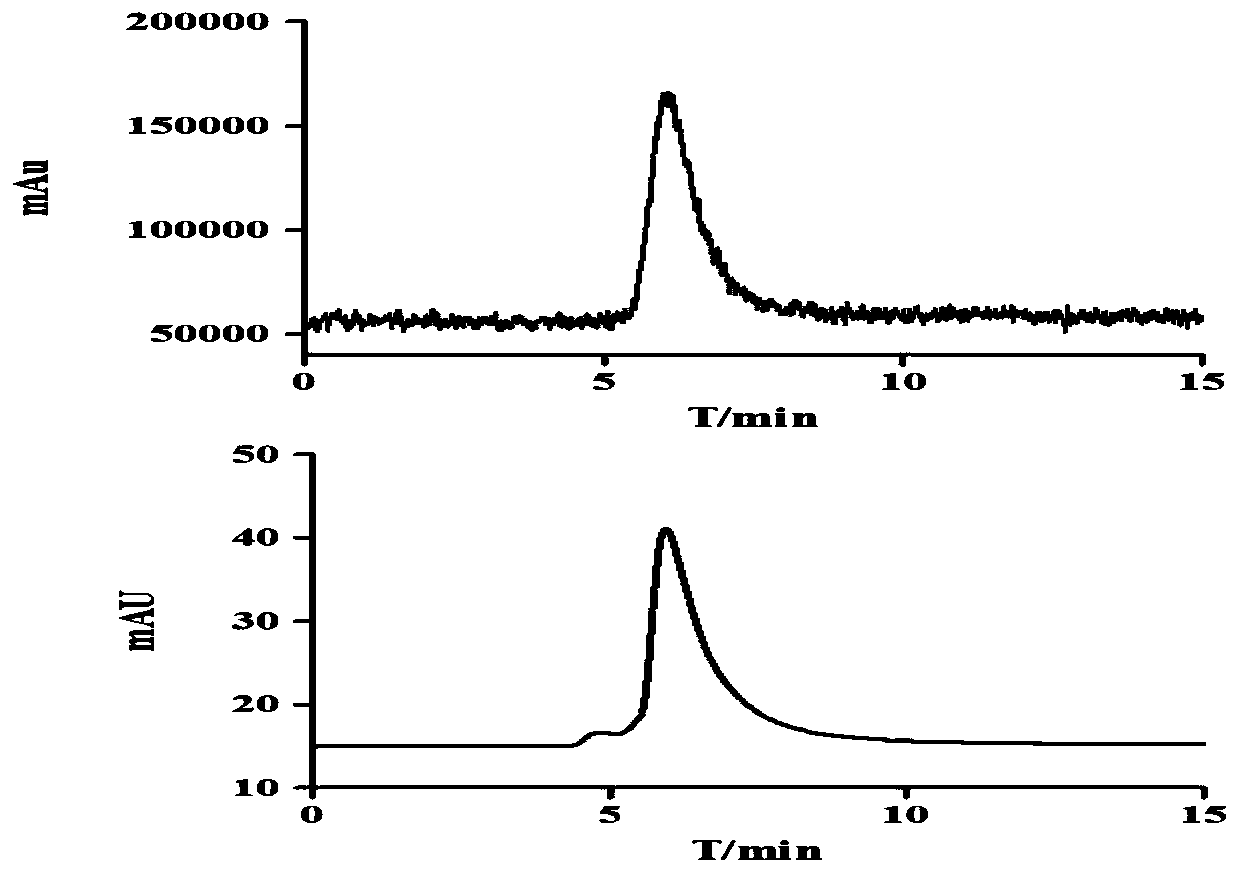
[0029] Radio-TLC and Radio-HPLC were used to determine the labeling rate and radiochemica...
Embodiment 2124I
[0030] Example 2 124 In vitro stability analysis of I-Trastuzumab labeled compounds
[0031] Stability in 0.01M pH 7.4PBS solution: take 10μL of purified radioactivity containing 37-74kBq (1-2μCi) 124 I-Trastuzumab was added to the 7.4PBS solution and incubated at 4℃ for 2h, 24h, 48h, 72h and 96h. Take out 2μL of the sample and mix it with 20ul of saturated EDTA. Take 2μL of the sample to be tested and drop it on the No. 1 filter paper from Xinhua Place the bottom 1cm in a physiological saline expansion system. After it is fully expanded, take out the filter paper and dry it for radio-TLC detection.
[0032] Stability in 5% HSA solution: Take 10μL of purified radioactivity containing 37-74kBq (1-2μCi) 124 I-Trastuzumab was added to 100ul of 5% HSA solution, and after incubating at 4℃ for 2h, 24h, 48h, 72h and 96h, 2μL of the sample was taken out and mixed with 20ul of saturated EDTA, and 2μL of the sample to be tested was dropped on Xinhuayi 1cm at the bottom of the No. filter pape...
Embodiment 3124I
[0034] Example 3 124 Study on PET imaging of I-Trastuzumab labeled compound in animals
[0035] Take 10 HER2-positive PDX models of gastric cancer randomly divided into 124 I-Trastuzumab positive group and 124 I-hIgG1 positive group, prepare 5 HER2-negative PDX models of gastric cancer (female, 5-6 weeks old, 18-20g, right inguinal area, tumor diameter up to 1cm), 124 I-Trastuzumab positive group and 124 I-Trastuzumab negative group was injected with 18.5MBq (0.5mCi, 200μL) via tail vein 124 I-Trastuzumab, 124 I-hIgG1 positive group was injected with 18.5MBq (0.5mCi, 200μL) via tail vein 124 I-hIgG1. Each PDX model in each group was subjected to Micro-PET imaging studies at 2h, 24h, 48h, 72h and 96h after injection; before imaging, nude mice were placed in the Summit AS-1-000-7 small animal anesthesia system at 3L / min. The mice are anesthetized with halothane gas, the PDX model is fixed in the center of the Micro-PET scanning bed in a prone position, and the mice are scanned in a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com