Recombinant human interferon alpha2a suppository and preparation method thereof
A technology of recombinant human interferon and interferon α, which is applied in suppository delivery, drug combination, pharmaceutical formula, etc., can solve the problems of poor patient compliance, protein instability, and high price, and achieve good therapeutic effect and preservation Long-term, stable biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Screening of Recombinant Human Interferon α2a Suppository Matrix Formula
[0037] The stock solution of recombinant human interferon α2a was prepared by our company (Changchun Institute of Biological Products Co., Ltd.), and reagents such as glycerin and gelatin were purchased commercially.
[0038] (1) Preliminary selection of suppository base
[0039] According to the literature and our laboratory's experience in the preparation of interferon suppositories, glycerin was initially selected as the excipient and bacteriostatic agent, and gelatin was used as the excipient and protease inhibitor.
[0040] (2) Preparation process of suppository base
[0041] 1) Matrix preparation and initial dissolution
[0042] Add glycerin, gelatin, and water for injection into a sterile and airtight suppository matrix tank according to the ratio of 5:2:1 to 5:3:2. Close the feed inlet valve, open the stirring switch, the exhaust valve, the circulating water valve and the ste...
Embodiment 2
[0055] Example 2 Preparation of Recombinant Human Interferon α2a Plug
[0056] The sources of various raw materials used in this embodiment are the same as those in Example 1.
[0057] (1) Matrix preparation and initial dissolution
[0058] Add glycerin, gelatin, and water for injection into a sterile and airtight suppository matrix tank according to the ratio of 5:2:1 to 5:3:2. Close the feed inlet valve, open the stirring switch, the exhaust valve, the circulating water valve and the steam valve, heat the temperature to 80-90°C, and stir continuously to make it dissolve, and the stirring speed is 20-40rpm.
[0059] (2) Matrix sterilization
[0060] Keep the stirring speed at 20-40rpm. Open the main steam valve of the suppository matrix tank, open the main exhaust valve, drain the condensed water, and close the main exhaust valve. Open the interlayer inlet valve of the matrix tank, open the interlayer exhaust valve, drain the condensed water in the interlayer, and close t...
Embodiment 3
[0071] Example 3 Stability investigation test of recombinant human interferon α2a plug
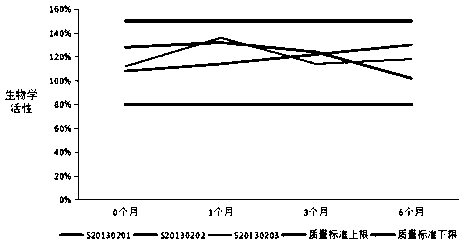
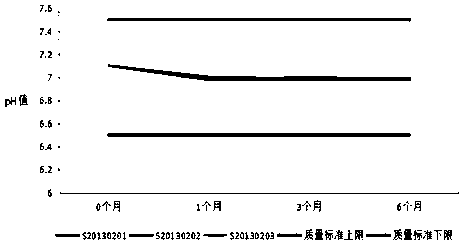
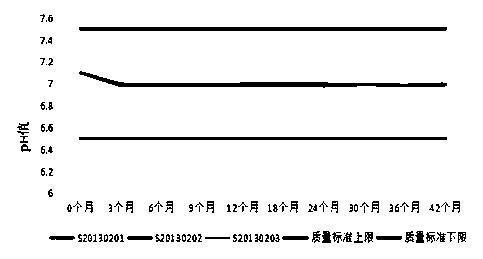
[0072] In order to further determine the stability of the formula and preparation method of the present invention, it is judged whether the drug is stable at the storage temperature to be changed by summarizing and analyzing the test data, so as to provide a theoretical basis for the determination of product packaging, transportation, storage conditions and expiration date , and reset the expiration date of the product during the stabilization period. Three batches of recombinant human interferon α2a suppositories produced continuously in 2013 (production batch numbers: S20130201, S20130202, and S20130203) were used as the research objects, and the accelerated stability test at 36.5-37.5°C and the long-term stability test at 29-31°C were carried out respectively.
[0073] 1. Accelerated stability investigation test of recombinant human interferon α2a suppository at 36.5-37.5°C:
[0074] A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com