Medical electrolyte composition and preparation method thereof
A composition and electrolyte technology, applied in the field of medical nutritious food and its preparation, can solve problems such as elevation, and achieve the effects of defecation and bowel cleansing, improvement and maintenance of living conditions, and reduction of fear and nervousness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
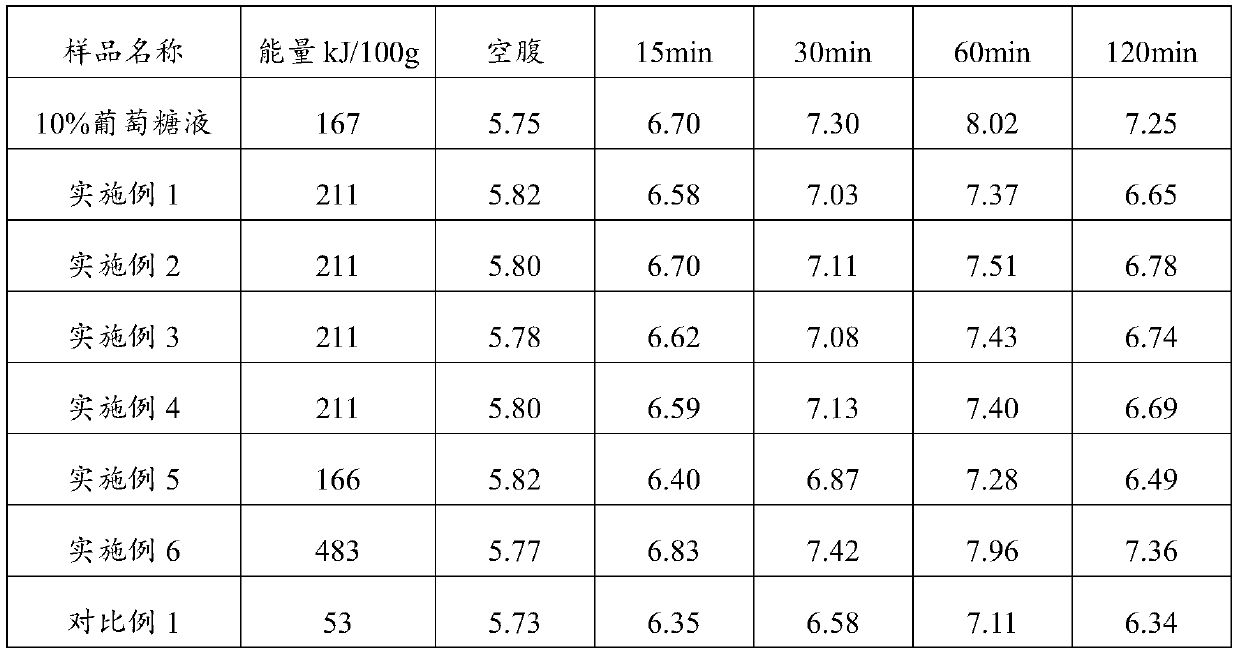
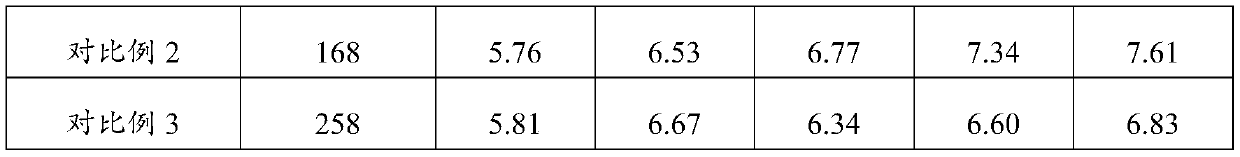
[0027] A medical electrolyte composition, the composition includes the following components in mass percentage: maltodextrin 9.8%, crystalline fructose 2.5%, galacto-oligosaccharide 1.5%, complex minerals (sodium citrate: potassium citrate = 1:2) 0.15%, citric acid 0.5%, food flavor 0.01%, the balance is water.
[0028] Its preparation method is:
[0029] (1) Pretreatment: the compound minerals and citric acid are weighed according to the proportion, and then water is added to completely dissolve to form a buffer solution;
[0030] (2) Weighing: Add maltodextrin, crystalline fructose, and oligosaccharides into the batching tank according to the proportion and stir evenly, then add buffer solution and stir evenly;
[0031] (3) Homogenization: Pass the prepared solution through a homogenizer;
[0032](4) Sterilization: use high temperature and short-term sterilization, the temperature is 125-135°C, the time is 15S, after cooling, add food flavor and stir evenly;
[0033] (5) ...
Embodiment 2
[0035] A medical electrolyte composition, said composition comprising the following components in mass percentage: 10.1% maltodextrin, 2.6% crystalline fructose, 0.4% xylooligosaccharide, complex minerals (sodium citrate: potassium citrate = 1:1) 0.15%, citric acid 0.5%, food flavor 0.01%, the balance is water.
[0036] Its preparation method is with embodiment 1.
Embodiment 3
[0038] A medical electrolyte composition, said composition comprising the following components in mass percentage: 9.8% maltodextrin, 2.6% crystalline fructose, 1.25% mannose oligosaccharides, complex minerals (sodium citrate: potassium citrate = 1:3) 0.15%, citric acid 0.5%, food flavor 0.01%, the balance is water.
[0039] Its preparation method is with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com