Electrochemical luminescence kit for detecting anti-LRP4 antibody and manufacturing method thereof
An electrochemical and kit technology, applied in the field of immunoassay, can solve the problems such as no method or kit for quantitatively detecting anti-LRP4 antibodies with high sensitivity and high specificity, and achieves fast detection speed, improved work efficiency, and linear range. wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation method of electrochemiluminescence kit for detecting anti-LRP4 antibody
[0023] 1) Preparation of working solution of magnetic beads coated with streptavidin
[0024] The suspension of streptavidin-coated magnetic bead particles was magnetically separated to remove the supernatant, and resuspended to a concentration of 0.75mg in PBS buffer (0.1M, pH 7.4) with pH=7.4 and a concentration of 0.1mol / L / mL, the buffer contains 0.5 wt% BSA, 0.05 wt% Triton X-100, 0.05 wt% proclin 300, and 0.05 wt% sodium azide.
[0025] 2) Preparation of biotin-labeled LRP4 antigen
[0026] Accurately weigh 1 mg of LRP4 antigen, add an appropriate amount of PBS buffer (0.1M, pH 7.4) to adjust the total antibody concentration to 1 mg / mL, and add it to a dialysis bag for dialysis. Change the dialysate every 3 to 4 hours. ~4 times, transfer the antibody to the centrifuge tube or cryopreservation tube after dialysis;
[0027] Accurately weigh 1 mg of N-hydroxysuccinimide-activated ...
Embodiment 2
[0043] Example 2 The method for detecting anti-LRP4 antibodies using the kit of the present invention uses an automatic electrochemiluminescence immunoassay analyzer as the detection instrument, and the kit is loaded on the instrument for detection. The steps are as follows:
[0044] Add the sample (or calibrator or quality control), biotin-labeled LRP4 antigen working solution and terpyridine ruthenium-labeled anti-human IgG antibody working solution to the reaction cup, and incubate at 37°C for 10 minutes to form antigen-antibody-antibody Antibody complex solution;
[0045] Add streptavidin-coated magnetic beads working solution to the antigen-antibody-anti-antibody complex solution, and incubate at 37°C for 10 minutes to form a magnetic complex suspension;
[0046] Placing the magnetic composite suspension in a magnetic field, and the cleaning fluid flows through and washing the magnetic composite;
[0047] Inject the electrochemiluminescence substrate solution into the washed magn...
Embodiment 3
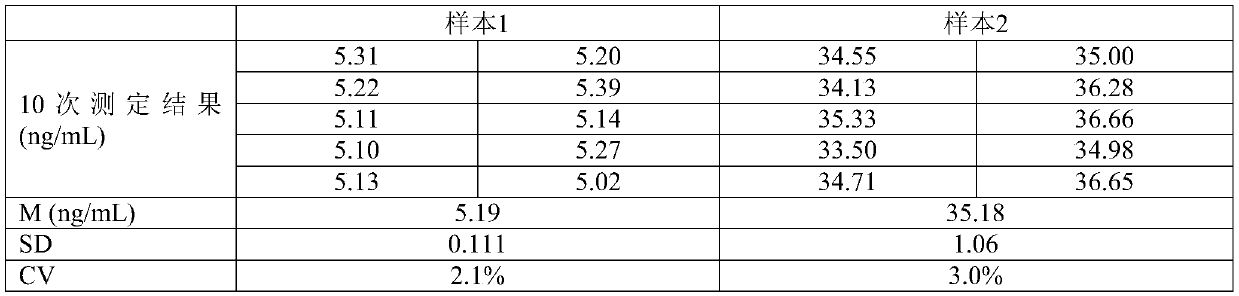
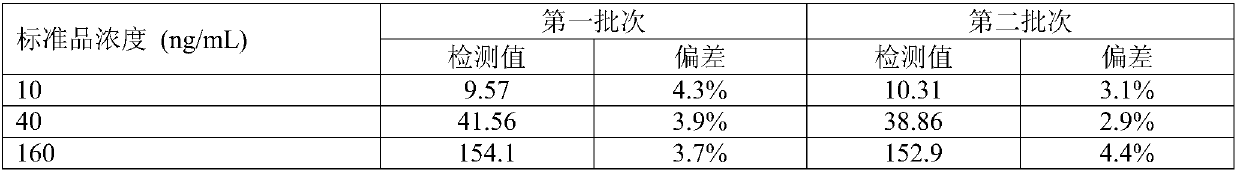
[0048] Example 3 Performance evaluation of the kit of the present invention
[0049] 1) Research on reagent sensitivity
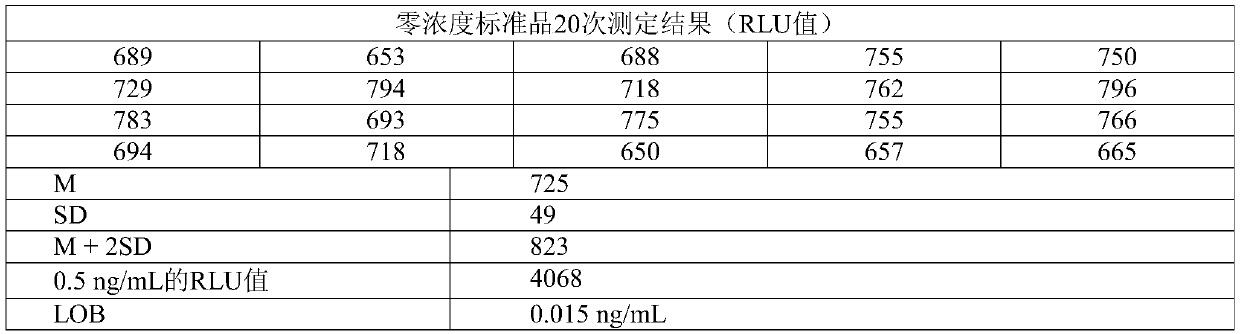
[0050] The reagent sensitivity is determined according to the minimum detection limit (Limit of Blank, LOB), and the minimum detection limit is performed according to the following experimental method. Check the zero-concentration calibrator 20 times, get the signal value (RLU) of the 20-time measurement results, calculate the average value M and standard deviation SD, and get the RLU value corresponding to M+2SD, according to the zero-concentration calibrator and the adjacent concentration The concentration between the calibrator (1ng / mL) and the average value of RLU are obtained by two-point regression fitting to obtain a linear equation. The RLU value corresponding to M+2SD is brought into the above equation to calculate the corresponding concentration, namely LOB .
[0051] The measurement results in Table 1 below show that the LOB of the reagent detected by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com