Application of gentiopicroside in preparation of medicines for treating hyperlipidemia
A technology of gentiopicroside and hyperlipidemia, which is applied in the field of biomedicine, can solve the problem of unreported blood lipid-lowering activity, and achieves the effect of relieving lipid accumulation and increasing the level.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
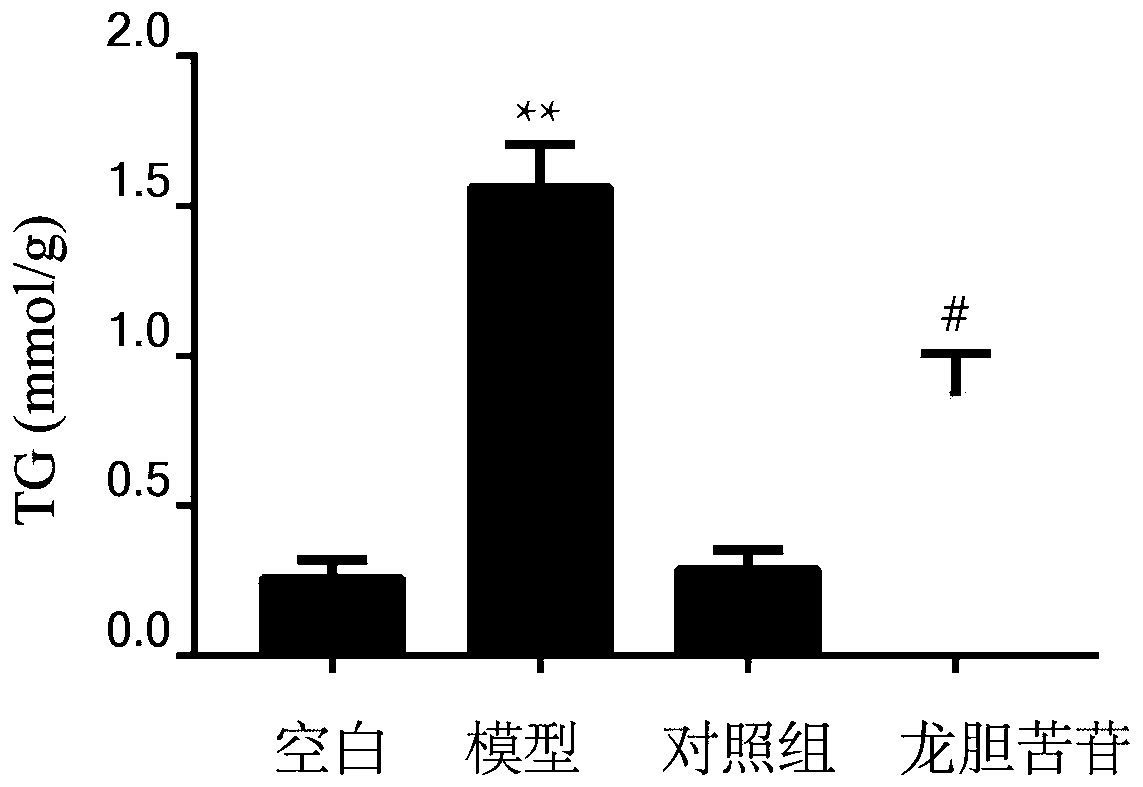
[0033] Determination of TG content in embodiment 1HepG2 cells
[0034] experimental method:
[0035] (1) HepG2 cells were seeded in a six-well plate with a density of 1×10 per well. 6 cells. The formula of the cell culture medium is DMEM medium with 4.5g / L glucose, 10% fetal bovine serum and 1% penicillin and streptomycin. Cells were cultured in 5% CO 2 and in a cell culture incubator at 37°C for 12 hours.
[0036] (2) Remove the original culture medium and replace it with DMEM with only 4.5g / L glucose as the culture medium to culture the cells, and starve the HepG2 cells for 3 hours.
[0037] (3) Incubate HepG2 cells with 200 μM gentiopicroside for 1 hour and then co-incubate the cells with 1000 μM free fatty acid (oleic acid:palmitic acid=2:1) for 24 hours; among them, divide into blank group (DMEM medium), model group (1000 μM free fatty acid), control group (200 μM gentiopicroside), experimental group (200 μM gentiopicroside + 1000 μM free fatty acid).
[0038] (4)...
Embodiment 2
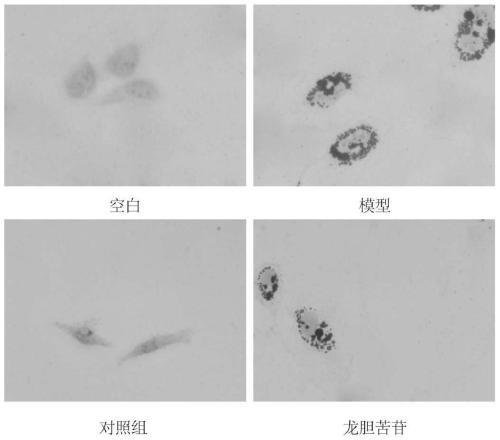
[0041] Example 2 HepG2 Cell Oil Red O Staining Experiment
[0042] experimental method:
[0043] (1)-(3) Experimental procedures are the same as in Example 1.
[0044] (4) Remove the cell culture medium, wash the cells 2-3 times with PBS, and then fix with 4% paraformaldehyde for 20-30 minutes.
[0045] (5) Formaldehyde was discarded, cells were washed 3 times with distilled water, and soaked with 60% isopropanol for 5 minutes.
[0046] (6) Stain with 0.5% Oil Red O staining solution for 10-20 minutes.
[0047] (7) Discard the staining solution, wash the cells 3-5 times with distilled water, and then stain with hematoxylin for 1-2 minutes.
[0048] (8) Observing lipid droplets with an optical microscope, the observation magnification is 1000 times.
[0049] (9) The experimental results are attached figure 2 .
[0050] attached figure 2 Oil red O staining results showed that the model group could increase the formation of lipid droplets in HepG2 cells, and the experime...
Embodiment 3
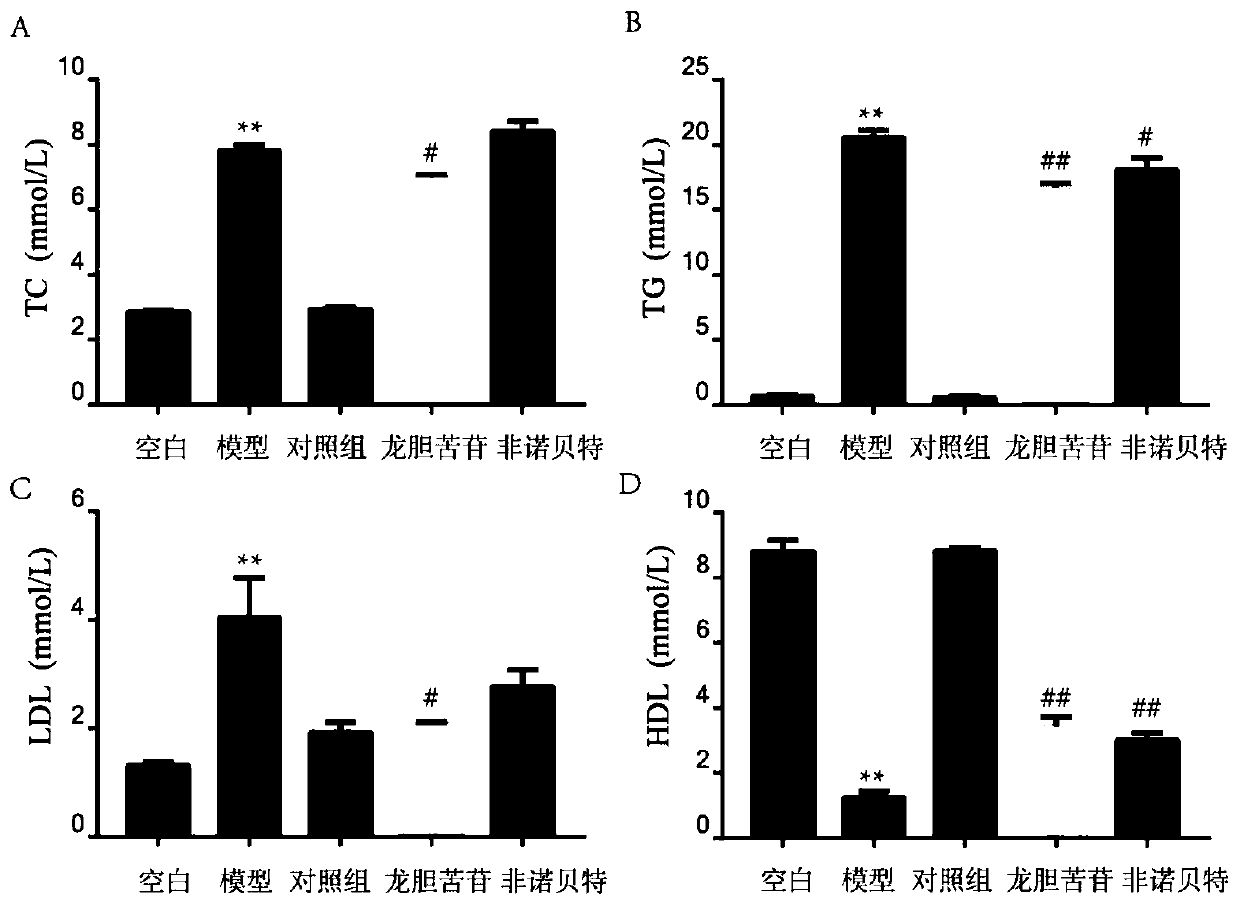
[0051] The mensuration of TC, TG, LDL, HDL content in the mouse serum of embodiment 3
[0052] experimental method:
[0053] (1) Raise the mice in an SPF environment with a temperature of 24±1° C. and a relative humidity of 40%-80%.
[0054](2) Before the experiment, the mice were fasted for 12 hours, then injected intraperitoneally with 40 mg / kg gentiopicroside for 1 hour, and then injected intraperitoneally with 500 mg / kg tyloxapol for 12 hours. The mice were randomly divided into five groups: control group (normal saline), model group (500 mg / kg tyloxapol), control group (40 mg / kg gentiopicroside), experimental group (40 mg / kg gentiopicrin Glycoside + 500 mg / kg Tyloxapol), drug positive control group (100 mg / kg fenofibrate + 500 mg / kg Tyloxapol).
[0055] (3) The mouse blood was obtained by taking blood from the eyeball, and left at room temperature. After the serum was precipitated, it was centrifuged at 3000 rpm for 5 minutes, and the upper layer of serum was absorbed. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com