Preparation method of lithium ion battery cathode material
A technology for lithium ion batteries and positive electrode materials, which is applied in the field of preparation of positive electrode materials for lithium ion batteries, can solve the problems of high production process requirements, complicated operations and the like, and achieves the effects of low cost, simple operation and good electrochemical performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
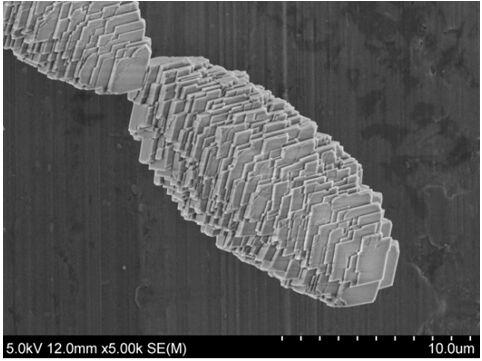
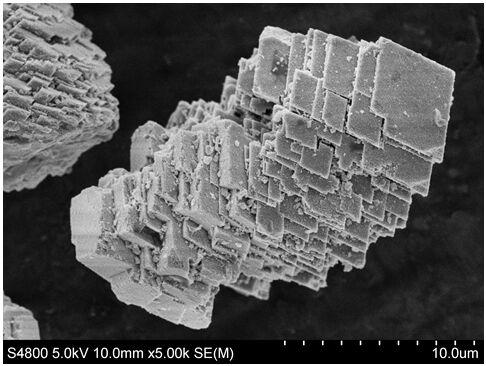
[0016] Weigh 2.9814g, 0.4901g, and 0.4981g of nickel acetate, cobalt acetate, and manganese acetate into a beaker, add 1g of CTAB into the beaker, then add 65mL of deionized water, stir at room temperature to dissolve the solid, and then add 2.5225g of urea for stirring until dissolved, transferred to the reaction kettle and heated at 200°C for 12 hours in a blast drying oven, then took it out, poured off the upper layer solution, washed the lower layer sediment with alcohol, centrifuged five times, and suction filtered three times with deionized water, then Transfer to a Petri dish and vacuum dry at 80°C for 12 hours to obtain Ni 0.8 co 0.1 mn 0.1 CO 3 Precursor powder. Weigh 1g of precursor powder and 0.3318g of lithium carbonate, mix and grind them thoroughly, and then calcinate in stages in a tube furnace under an oxygen atmosphere. Carry out the second calcination, the temperature is 800°C, the heating rate is 3°C / min, and the time is 12 hours, then LiNi is obtained. ...
Embodiment 2
[0018] Weigh 2.9814g, 0.4901g, and 0.4981g of nickel acetate, cobalt acetate, and manganese acetate into a beaker, add 1.5g of CTAB into the beaker, and then add 65mL of deionized water, stir at room temperature to dissolve the solid, and then add 2.5225g of urea to carry out Stir until dissolved, transfer to the reaction kettle, heat in a blast drying oven at 200°C for 12 hours, take it out, pour off the upper solution, wash the lower sediment with alcohol, centrifuge five times, and filter three times with deionized water. Then transfer to a Petri dish and vacuum dry at 80°C for 12 hours to obtain the precursor powder Ni 0.8 co 0.1 mn 0.1 CO 3 . Weigh 1g of precursor powder and 0.3318g of lithium carbonate, mix and grind them thoroughly, and then calcinate in stages in a tube furnace under an oxygen atmosphere. Carry out the second calcination, the temperature is 800°C, the heating rate is 3°C / min, and the time is 12 hours, then LiNi is obtained. 0.8 co 0.1 mn 0.1 o ...
Embodiment 3
[0020] Weigh 2.9814g, 0.4901g, and 0.4981g of nickel acetate, cobalt acetate, and manganese acetate into a beaker, add 2g of CTAB into the beaker, then add 65mL of deionized water, stir at room temperature to dissolve the solid, and then add 2.5225g of urea for stirring until dissolved, transferred to the reaction kettle and heated at 200°C for 12 hours in a blast drying oven, then took it out, poured off the upper layer solution, washed the lower layer sediment with alcohol, centrifuged five times, and then suction filtered three times with deionized water, Then transfer to a Petri dish and vacuum dry at 80°C for 12 hours to obtain the precursor powder Ni 0.8 co 0.1 mn 0.1 CO 3 . Weigh 1g of precursor powder and 0.3318g of lithium carbonate, mix and grind them thoroughly, and then calcinate in stages in a tube furnace under an oxygen atmosphere. Carry out the second calcination, the temperature is 800°C, the heating rate is 3°C / min, and the time is 12 hours, then LiNi is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com