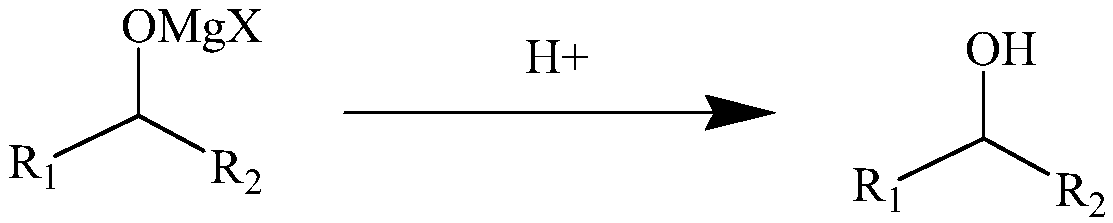Environmentally friendly hydrolysis process of Grignard reaction metal organic product
A metal-organic and Grignard reaction technology, applied in organic chemistry, hydrolysis preparation, magnesium sulfate, etc., can solve the problems of high cost, adverse effect of Grignard reaction yield, etc., and achieve the effect of zero waste water discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
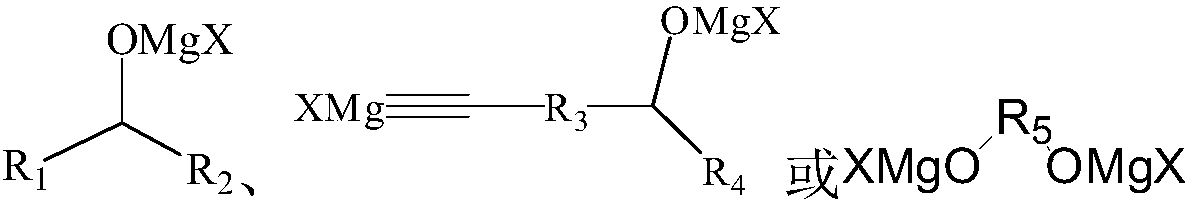
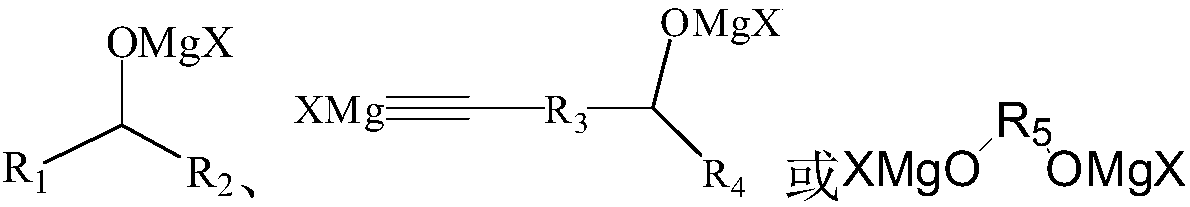
[0038] The metal organic product obtained by the Grignard reaction is called dichloromagnesium condensate for short, and the structure is as follows:
[0039]
[0040] Add 55.81g (0.315mol) pyridine hydrogen sulfate solid to the four-neck reactor, add 100ml ether solution and stir to cool down to about 10℃, then add the Grignard reaction dropwise to obtain the 0.75mol / L dichloromagnesium condensate 300ml of ether solution (containing 0.225mol of bischloromagnesium condensate), drip it in about 20 minutes, and then continue to stir for 90 minutes to stop the hydrolysis reaction. Add excess magnesium hydroxide to complete the reaction of excess pyridine hydrogen sulfate, and then filter to obtain 65 g of white solid and 394 ml of ether solution of the condensate product.
[0041] The solid is washed with 100ml of pure ether to remove a small amount of condensate. The washing liquid can be used as the solvent for the next pyridine bisulfate solid dissolution; the solids are mainly mag...
Embodiment 2
[0049] The metal-organic product obtained by the Grignard reaction is referred to as bis-bromomagnesium condensate, and the structure is as follows:
[0050]
[0051] Add 53.82g (0.21mol) of pyridine sulfate solid to the four-neck reactor, add 100ml of methyltetrahydrofuran solution and stir to cool down to about -20℃, then add the Grignard reaction dropwise to obtain a 1mol / L bisbromomagnesium condensate 200ml of methyl tetrahydrofuran solution (containing 0.2mol of bis-bromomagnesium condensate), drip in about 90 minutes, and then continue to stir for 20 minutes to stop the hydrolysis reaction. Adding excess magnesium hydroxide to complete the reaction of excess pyridine hydrogen bromide, and then filtering to obtain 65.5 g of white solid and 295 ml of methyl tetrahydrofuran solution as a condensate product.
[0052] The solid is washed with 100ml pure methyltetrahydrofuran to remove a small amount of condensate. The washing liquid can be used as the solvent for the next pyridine...
Embodiment 3
[0060] The metal organic product obtained by the Grignard reaction is trityl magnesium bromide, the structure is as follows:
[0061]
[0062] Add 52.2g (0.3mol) of 3-methylpyridine hydrobromide solid to the four-neck reactor, add 100ml of tetrahydrofuran solution and stir and raise the temperature to about 50℃, then add the Grignard reaction to obtain a concentration of 2.5mol / L. 100ml of tetrahydrofuran solution of benzylmagnesium bromide (containing 0.25mol of tritylmagnesium bromide), drip it in about 60min, and then continue to stir for 30min before stopping the hydrolysis reaction. Excessive magnesium hydroxide was added to completely react the excess 3-methylpyridine hydrobromide, and then filtered to obtain 51 g of white solid and 220 ml of triphenylmethane tetrahydrofuran solution.
[0063] The solid is washed with 100ml pure tetrahydrofuran to remove a small amount of triphenylmethane. The washing liquid can be used as the solvent for the next 3-methylpyridine hydrobromid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com