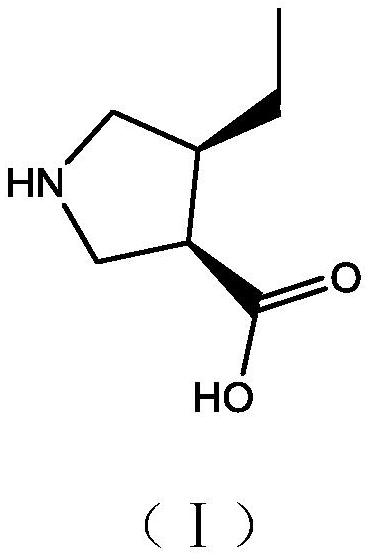
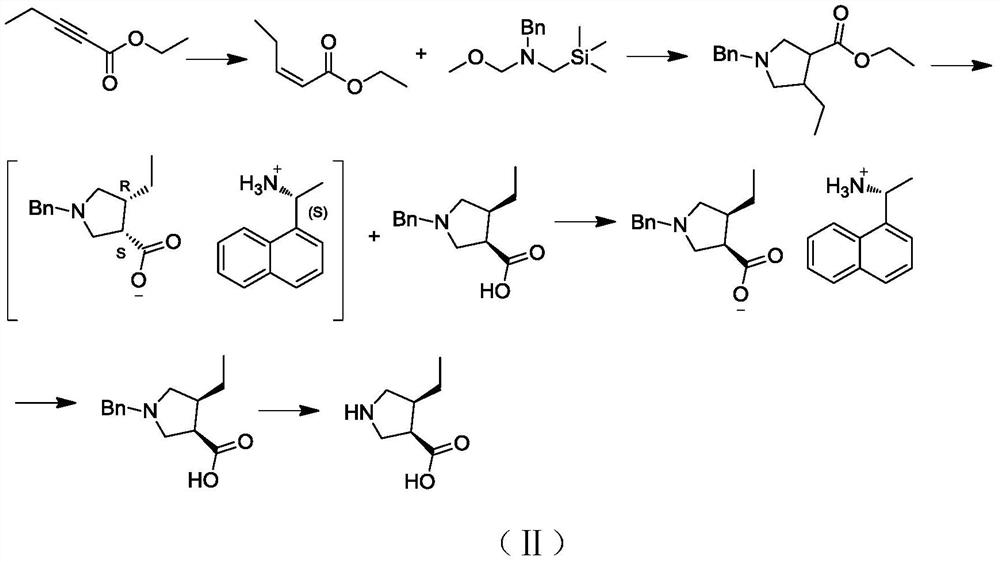
A kind of preparation method and application of (3r,4s)-4-ethylpyrrolidine-3-carboxylic acid compound
An ethylpyrrolidine and compound technology, applied in the field of pharmaceutical synthesis, can solve problems such as low yield and high cost, and achieve the effects of simple synthesis route, low synthesis cost, and simple and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
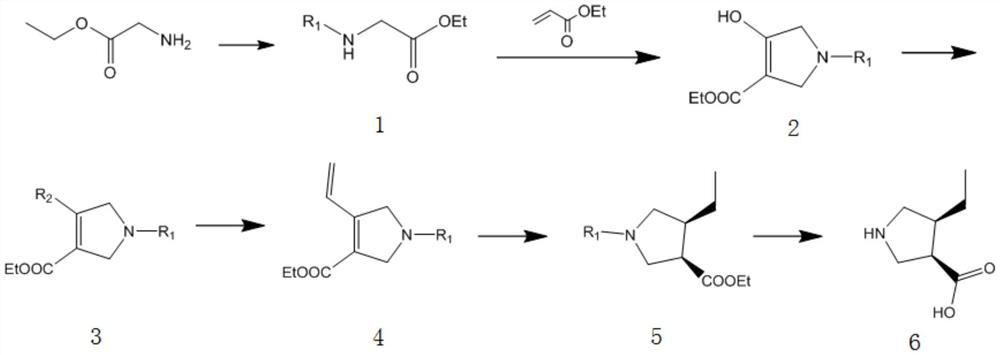
[0046] (3R, 4S) -4- ethyl-pyrrolidine-3-carboxylic acid compounds, comprising the steps of:
[0047] (1) Synthesis of Compound 1
[0048] Its reaction equation is as follows:
[0049]
[0050]73.8 g of glycine ethyl ester was dissolved in 500 ml of dichloromethane in 1000 ml of reaction flask, and 144.8 g of triethylamine was added dropwise thereto at 0 ° C, and the dropwise addition was added, and 74.52 g of methyl chloroformate was added dropwise. After the reaction was allowed to at room temperature, after 3 h, 100 ml of aqueous hydrochloric acid solution (1 m), a liquid, and the organic phase was sequentially washed with saturated brine and water, dried over anhydrous sodium sulfate, filtered, evaporated. The compound 1 was obtained directly for the next reaction.
[0051] (2) Synthetic Compound 2
[0052] Its reaction equation is as follows:
[0053]
[0054] The compound 1 obtained by step (1) is dissolved in 400 ml of tetrahydrofuran, then 72 g of ethyl acrylate, drown ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com