Recombinant short peptide, production method and application thereof, and application of recombinant short peptide in promoting wound healing
A production method and short peptide technology, applied in the production method and application, and the field of recombinant short peptides, to achieve the effect of solving delayed wound healing and non-healing and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Screening and Synthesis of Genes
[0029] Firstly, codon optimization was carried out on the gene sequence of rEGF, and at the same time, Escherichia coli preferred stop codon TAA was added to the 3' end, and the gene sequence as shown in SEQ ID NO:1 was designed, specifically as follows:
[0030] ATTAGCACAAAATAATGGGAATAAAGGGAACAGTTCTGAACCAAACTGCTGACATTGGGCTCCTGTCCTGGCTCTCCTGTAAAGGAAATTACATGGATGAGGAGGTCCAAGTCTCTGTCACCAGGGAGCACTCACTGGAATGGGTGAGCAGAACCCTAGGGTATTAGAAGGCAGGACTGAGTTCTAATTTTCCTGGGTCCCCAGCACCTGAAACTGTCAAGTTCAT.
[0031] The length of the nucleic acid is 237bp, and a third-party company is commissioned to synthesize the DNA sequence of the gene.
Embodiment 2
[0032] Example 2 Construction and Screening of Escherichia coli Transformants
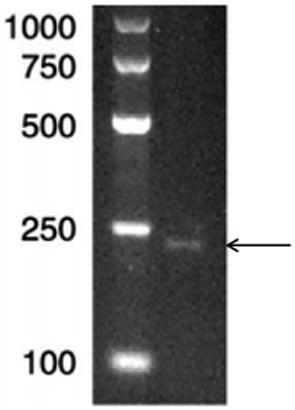
[0033] The synthesized vector pUC-rECF containing the gene sequence of SEQ ID NO: 1 was digested with restriction endonucleases Nde I and Xho I at 37°C for 1 h, and the small fragments after digestion were recovered on agarose gel to obtain the 240bp target fragment, see figure 1 . Ligate with the expression vector pET-24b that has been cut with the same double enzymes under the action of T-DNA ligase at 16°C for 1 hour, and transform the ligated product into competent Escherichia coli BL21 by calcium chloride method. Cultivate in culture medium and select positive bacteria.
[0034] The construction of the pET-24b-rEGF recombinant plasmid, the connection system is as follows:
[0035] pET-24b digested empty vector 1 μL
[0036] 2.5 μL of target gene after enzyme digestion
[0037] T4 DNA Ligase 1μL
[0038] T4 DNA Ligase Buffer 2.5μL
[0039] Add dd H 2 0 to 25 μL.
[0040] For the specif...
Embodiment 3
[0042] Example 3 induced expression
[0043] Inoculate the positive strains in the LB liquid medium containing kanamycin, shake at 200 r / min at 37°C for overnight activation, and re-inoculate the LB medium at a ratio of 1:100 to detect the growth density of the bacteria. D. 600 When it reached 0.4, IPTG was added to make the final concentration 0.3 mmol / L, and the expression was induced at 37°C for 7 hours, and the precipitate was collected by centrifugation to obtain the bacteria.
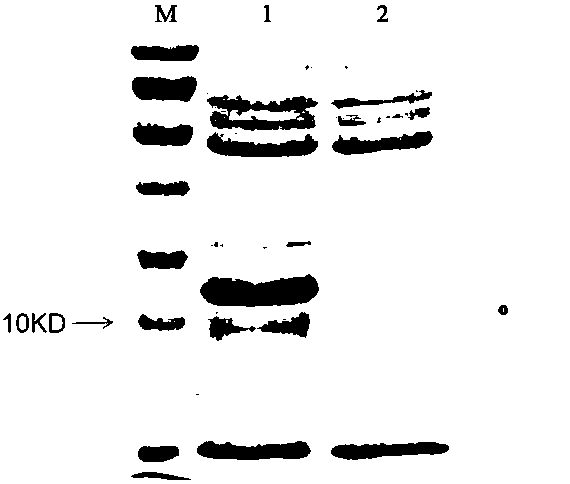
[0044] Protein expression detection: take the bacteria collected in step (3), add PBS buffer and mix well, break up by ultrasonic in an ice bath, add 5× protein loading buffer to the expression bacteria solution, boil and take 20uL for 15% SDS-PAGE (recipe See Table 1) Detection. Analyze the expression level of the target protein in the prokaryotic system, as shown in the attached figure 2 As shown, compared with the control bacteria (the Escherichia coli strain containing the blank expression...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com