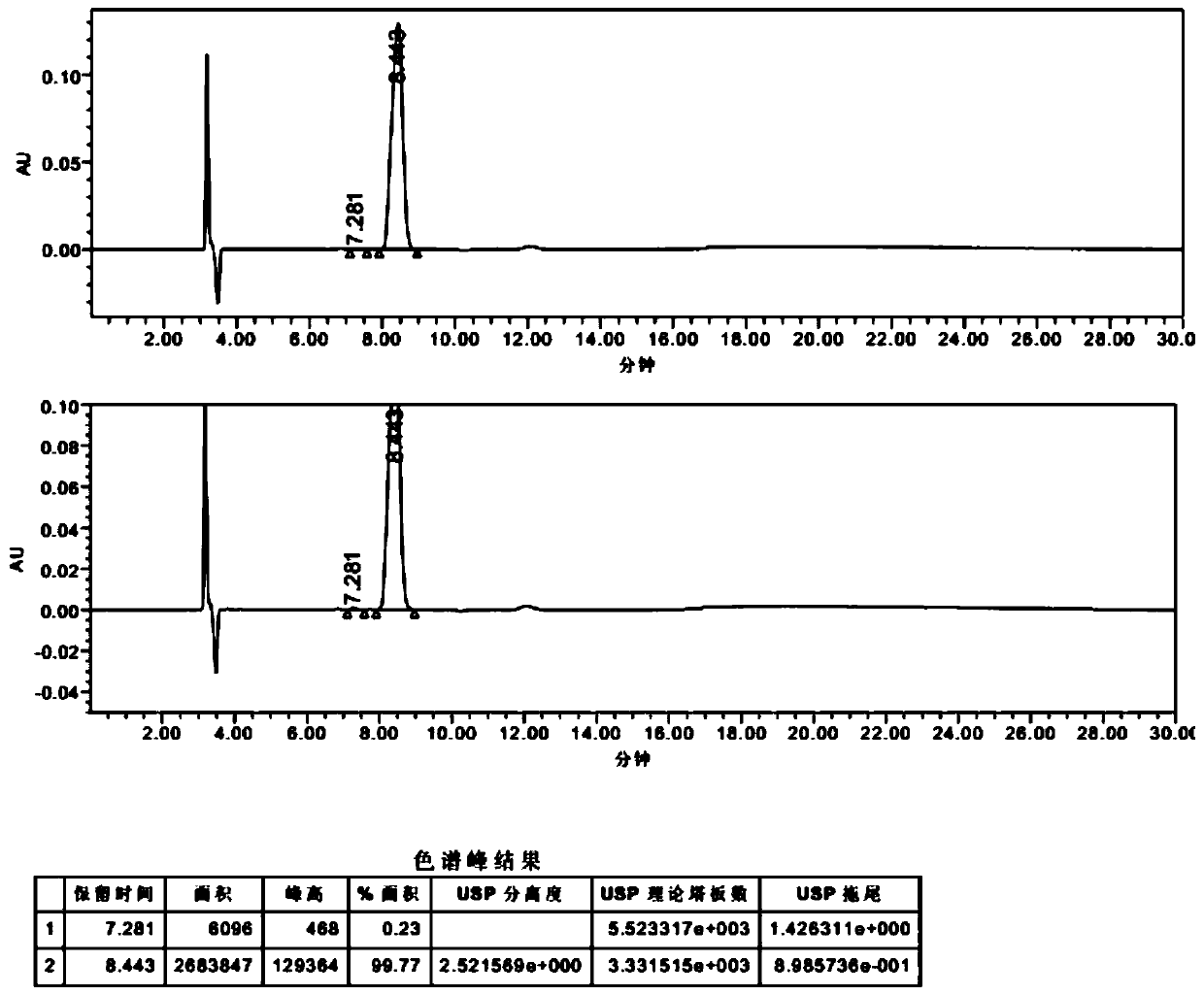
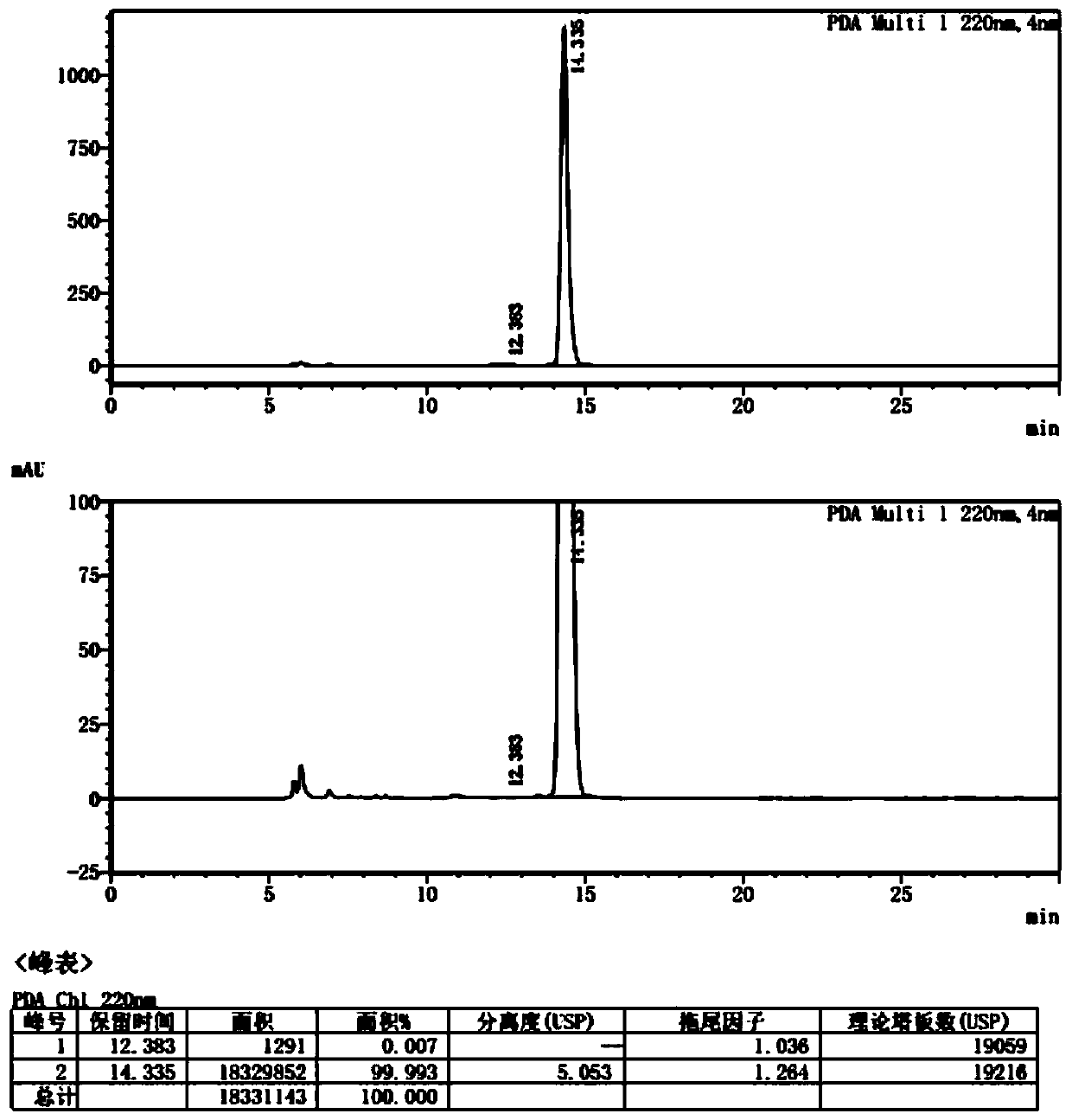
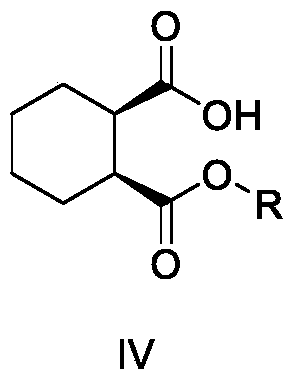
Preparation method of trans-1,2-cyclohexanedimethanol
A compound and selected technology, applied in the field of pharmaceutical intermediates, can solve the problems of low resolution yield, low photochemical purity, a large amount of waste liquid, etc., and achieve the effect of improving yield and photochemical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057]
[0058] Preheat and melt cis-hexahydrophthalic anhydride (220Kg, 1.427Kmol), then dissolve and dilute it with methanol (522Kg) and add it to the reaction kettle, slowly add concentrated sulfuric acid (28Kg, 0.285Kmol) dropwise, and heat to 65-70°C after dropping Reflux for 12 hours, add sodium bicarbonate to adjust the pH to 7-8, collect the filtrate by filtration, add water (220Kg), then extract with dichloromethane (874Kg), concentrate under reduced pressure to dryness to obtain the oil cyclohexane-1, Dimethyl 2-dicarboxylate III-a 280Kg, yield 98%.
Embodiment 2
[0060]
[0061]Add cis-hexahydrophthalic anhydride (500g, 3.244mol) into the reaction flask, add ethanol (1.5L) to dissolve and disperse, slowly add concentrated sulfuric acid (64g, 0.65mol) dropwise, and heat to 75-80°C under reflux for reaction 16 hours, add sodium bicarbonate to adjust the pH to 7-8, collect the filtrate by filtration, add water (500mL), extract with dichloromethane (1.5L), and concentrate under reduced pressure to dryness to obtain oil cyclohexane-1,2- Diethyl dicarboxylate III-b 688g, yield 93%.
Embodiment 3
[0063]
[0064] Add cis-hexahydrophthalic anhydride (100g, 0.648mol) into the reaction flask, add isopropanol (300mL) to dissolve and disperse, slowly add concentrated sulfuric acid (12.7g, 0.13mol) dropwise, and heat to reflux at 70-85°C after dropping React for 24 hours, add sodium bicarbonate to adjust the pH to 7-8, collect the filtrate by filtration, add water (200mL), then extract with dichloromethane (600mL), concentrate under reduced pressure to dryness to obtain oily cyclohexane-1,2 -Diisopropyl dicarboxylate III-c 134g, yield 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com