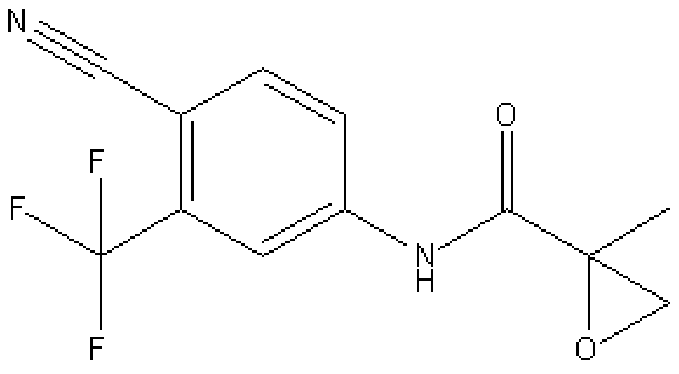
Synthesis method of N-(4-cyano-3-(trifluoromethyl)phenyl)-2-methyl epoxypropylene-2-amide
A technology of methyl epoxy propylene and trifluoromethyl phenyl is applied in the field of synthesis of N-phenyl)-2-methyl epoxy propylene-2-amide, which can solve the instability of peroxide and processing cost problems such as rising and environmental unfriendliness, and achieve the effect of high safety in production operation, low environmental pollution, and simple and easy post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
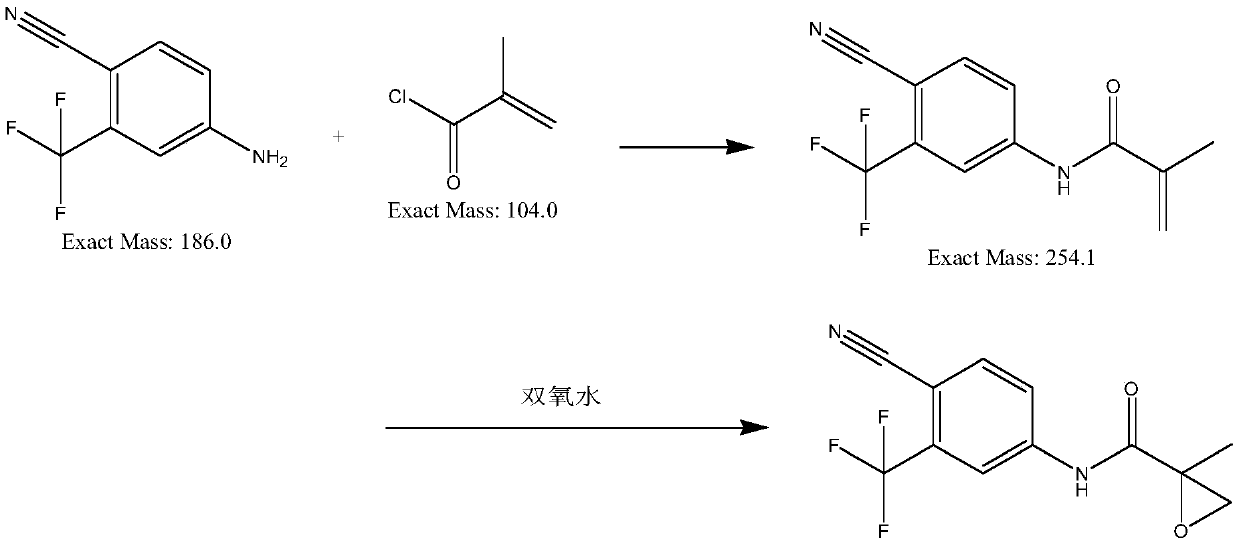
[0041] Example 1: In a 500L reactor, 18.6kg of 4-amino-2-trifluoromethylbenzonitrile and 19.9kg of methyl methacrylate and 200kg of dichloromethane and 100g of DMF were heated up by 30 degrees , reacted for 3h, pulled dichloromethane dry, and added 200kg of water into a 500L reactor, cooled to 20 degrees and filtered to obtain 23kg of light yellow solid, which was intermediate N-(4-cyano-3- Trifluoromethylphenyl)methacrylamide;
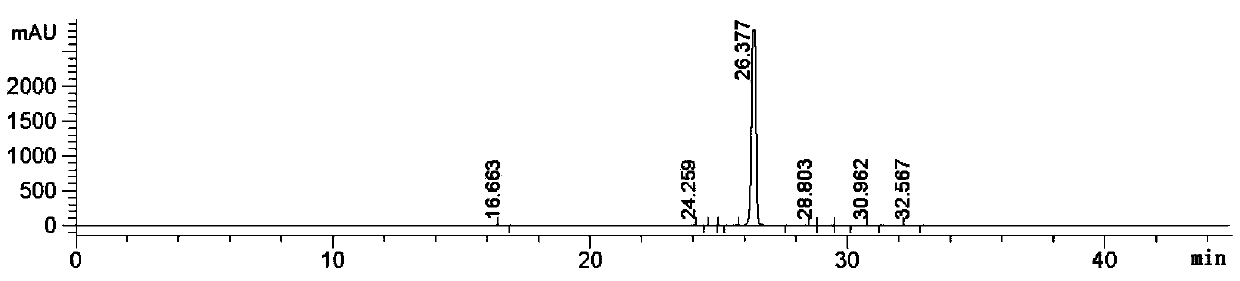
[0042] Add the above-mentioned 23kg of intermediates, 230kg of water, 2.3kg of tetrabutylammonium bromide and 0.23kg of manganese dioxide into a 500L reactor, stir for 0.5h and raise the temperature to 47 degrees, keep 47 degrees, pass into Air, kept at 45-50 degrees for 12 hours, cooled to 20-25 degrees, filtered, dried to obtain 24kg of N-(4 cyano-3-(trifluoromethyl)phenyl)-2-methylepoxypropene -2-amide, yield 98%, purity 99.42%.
Embodiment 2
[0043] Example 2: In a 500L reactor, 18.6kg of 4-amino-2-trifluoromethylbenzonitrile and 19.9kg of methyl methacrylate and 200kg of toluene and 100g of DMF were heated up by 30 degrees to react 3h, pull the toluene dry, and add 200kg of water into a 500L reaction kettle, cool down to 20 degrees and filter to obtain 17kg of light yellow solid, which is the intermediate N-(4-cyano-3-trifluoromethyl Phenyl)methacrylamide;
[0044] Add the above-mentioned 17kg of intermediates, 170kg of water, 1.7kg of tetrabutylammonium bromide and 0.17kg of manganese dioxide into a 500L reactor, stir for 0.5h and raise the temperature to 47 degrees, keep 47 degrees, pass into Air, kept at 45-50 degrees for 12 hours, cooled to 20-25 degrees, filtered, dried to obtain 17kg of N-(4 cyano-3-(trifluoromethyl)phenyl)-2-methylepoxypropene -2-amide, yield 69.4%, purity 87.62%.
Embodiment 3
[0045]Example 3: In a 500L reactor, 18.6kg of 4-amino-2-trifluoromethylbenzonitrile and 19.9kg of methyl methacrylate and 200kg of dichloromethane and 100g of DMF were heated up by 30 degrees , reacted for 3h, pulled dichloromethane dry, and added 200kg of water into a 500L reactor, cooled to 20 degrees and filtered to obtain 23kg of light yellow solid, which was intermediate N-(4-cyano-3- Trifluoromethylphenyl)methacrylamide;
[0046] Add the above-mentioned 23kg of intermediates, 230kg of water, 1.5kg of tetrabutylammonium bromide and 0.15kg of manganese dioxide into a 500L reactor, stir for 0.5h and raise the temperature to 47 degrees, keep 47 degrees, pass into Air, kept at 45-50 degrees for 12 hours, cooled to 20-25 degrees, filtered, dried to obtain 11kg of N-(4 cyano-3-(trifluoromethyl)phenyl)-2-methylepoxypropene -2-amide, yield 44.4%, purity 91.42%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com