Preparation method for synthesizing pyrroloquinoline quinone by four-step method
A technology of pyrroloquinoline quinone and four-step method, which is applied in organic chemistry and other fields, can solve the problems of high preparation cost, large environmental pollution, and difficult industrial production, and achieve the effect of simple method, environmental friendliness, and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
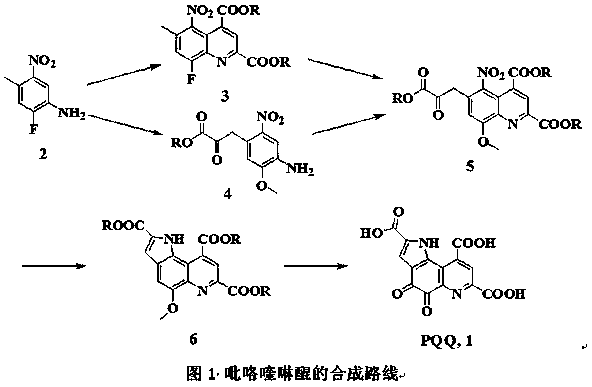
[0018] The preparation method of pyrroloquinoline quinone: 4-methyl-5-nitro-2-fluoroaniline (2) (170.14 g, 1.00 mol), 1,2-dichloroethane (1.00 L), 4-oxo Diethyl-2-enylglutarate (200.19 g, 1.00 mol) and concentrated hydrochloric acid (8 mL, 0.1 mol) were placed in a three-necked flask, and heated to reflux for 5 h. After the reaction was cooled, the liquids were separated and dried. The solvent was recovered under reduced pressure to obtain the crude product 6-methyl-5-nitro-8-fluoroquinoline-2,4-dicarboxylic acid diethyl ester (3); compound 3, diethyl oxalate (146.14 g, 1.00 mol), sodium methoxide (54.02 g, 1.00 mol) and methanol (1.00 L) were placed in a three-necked flask, heated to reflux for 5 h, and after the reaction was cooled, filtered to obtain 6-(2-ethoxycarbonyl-2-oxo Diethyl)-5-nitro-8-methoxyquinoline-2,4-dicarboxylate (5) in methanol; compound 5 in methanol and 5% palladium on carbon (2 g) Place in a high-pressure reactor, fill with hydrogen, keep the pressure a...
Embodiment 2
[0020] The preparation method of pyrroloquinoline quinone: 4-methyl-5-nitro-2-fluoroaniline (2) (170.14 g, 1.00 mol), dichloromethane (1.00 L), 4-oxo-2-ene Dimethyl glutarate (172.14 g, 1.00 mol) and 5 M dilute sulfuric acid (20 mL, 0.1 mol) were placed in a three-neck flask, heated to reflux for 5 h, after the reaction was cooled, separated, dried, and recovered under reduced pressure solvent to obtain crude 6-methyl-5-nitro-8-fluoroquinoline-2,4-dicarboxylic acid dimethyl ester (3); compound 3, dimethyl oxalate (118.09 g, 1.00 mol), Sodium carbonate (212.00 g, 2.00 mol) and methanol (1.00 L) were placed in a three-necked flask, heated to reflux for 5 h, and after the reaction was cooled, filtered to obtain 6-(2-methoxycarbonyl-2-oxoethyl )-5-nitro-8-methoxyquinoline-2,4-dicarboxylate (5) in methanol; compound 5 in methanol and Raney nickel (5 g) were subjected to high pressure reaction In the kettle, fill with hydrogen, keep the pressure of 5 atmospheres, heat to 50 ° C, re...
Embodiment 3
[0022] The preparation method of pyrroloquinoline quinone: 4-methyl-5-nitro-2-fluoroaniline (2) (170.14 g, 1.00 mol), benzene (1.00 L), 4-oxo-2-pentadiene Acetate dibenzyl ester (324.33 g, 1.00 mol) and methanesulfonic acid (9.61 g, 0.1 mol) were placed in a three-necked flask, and heated to reflux for 5 h. After the reaction was cooled, the liquid was separated, dried, and the solvent was recovered under reduced pressure to obtain Crude dibenzyl 6-methyl-5-nitro-8-fluoroquinoline-2,4-dicarboxylate (3); compound 3, dibenzyl oxalate (270.28 g, 1.00 mol), potassium carbonate (276.00 g, 2.00 mol) and methanol (1.00 L) were placed in a three-necked flask, heated to reflux for 5 h, and after the reaction was cooled, filtered to obtain 6-(2-benzyloxycarbonyl-2-oxoethyl)- Dibenzyl 5-nitro-8-methoxyquinoline-2,4-dicarboxylate (5) in methanol; compound 5 in methanol and 20% platinum on carbon (5 g) were placed in an autoclave , filled with hydrogen, kept at a pressure of 5 atmospheres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com