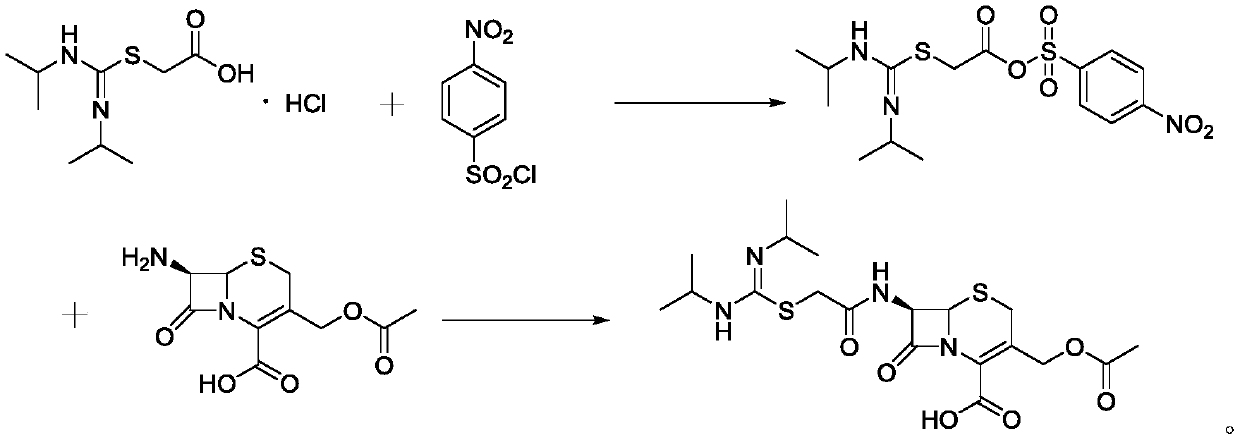
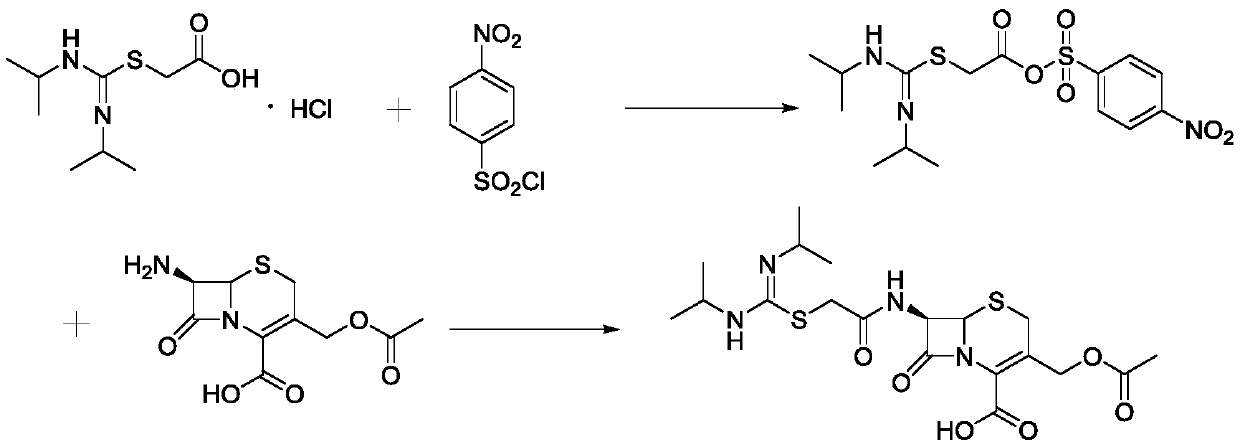
Synthesis process of cefathiamidine
A technique for cefathiamidine and synthesis process, which is applied in the field of cefathiamidine synthesis process, can solve problems such as unsatisfactory operation, complicated operation, and high price, and achieve the effects of promoting smooth progress, high yield and purity, and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Add 60ml of dichloromethane to a 250mL reaction flask, add 10.17g of 1,3-diisopropylamidino-2-thioacetic acid hydrochloride, add 8.90g of 4-pyrrolidinylpyridine, and add Sodium 1.76g, lower the temperature to 0-10°C, slowly add the dichloromethane solution of p-nitrobenzenesulfonyl chloride (9.72g dissolved in 30ml methylene chloride) dropwise, the dropwise addition is completed in about 15 minutes, control the temperature at 0-10°C and stir for 1h , HPLC detected that the reaction was substantially complete, and filtered to obtain filtrate A.
[0031] (2) Add 2.92g of N,N-dimethylformamide to filtrate A, slowly add 10.88g of 7-ACA, dropwise add 30% sodium hydroxide solution to adjust the pH to 4.5, stir for 15min, add dropwise 30% hydroxide Adjust the pH to 4.5 with sodium solution, stir for 15 minutes, add dropwise 30% sodium hydroxide solution to adjust the pH to 4.5, stir at room temperature for 2 hours, monitor the end of the reaction by HPLC, distill under red...
Embodiment 2
[0033] (1) Add 60ml of dichloromethane to a 250mL reaction flask, add 10.17g of 1,3-diisopropylamidino-2-thioacetic acid hydrochloride, add 9.77g of 4-dimethylaminopyridine, and add Potassium 2.69g, lower the temperature to 0-10°C, slowly add the dichloromethane solution of p-nitrobenzenesulfonyl chloride (9.72g dissolved in 30ml methylene chloride) dropwise, the dropwise addition is completed in about 15 minutes, control the temperature at 0-10°C and stir for 1h , HPLC detected that the reaction was substantially complete, and filtered to obtain filtrate A.
[0034] (2) Add 3.48g of N,N-dimethylacetamide to filtrate A, slowly add 10.88g of 7-ACA, add dropwise 30% sodium hydroxide solution to adjust the pH to 5.0, stir for 15min, add dropwise 30% hydroxide Adjust the pH to 5.0 with sodium solution, stir for 15 minutes, add dropwise 30% sodium hydroxide solution to adjust the pH to 5.0, stir at room temperature for 2 hours, monitor the end of the reaction by HPLC, distill under...
Embodiment 3
[0036] (1) Add 60ml of dichloromethane to a 250mL reaction flask, add 10.17g of 1,3-diisopropylamidino-2-thioacetic acid hydrochloride, add 7.72g of 2,6-lutidine, add Sodium carbonate 6.36g, lower the temperature to 0-10°C, slowly add p-nitrobenzenesulfonyl chloride dichloromethane solution (9.72g dissolved in 30ml methylene chloride) dropwise, the dropwise addition is completed in about 15 minutes, control the temperature at 0-10°C and stir After 1 h, the reaction was detected by HPLC to be almost complete, and filtered to obtain filtrate A.
[0037] (2) Add 5.17g of N,N-diisopropylethylamine to filtrate A, slowly add 10.88g of 7-ACA, add dropwise 30% sodium hydroxide solution to adjust the pH to 5.5, stir for 15min, and dropwise add 30% hydrogen Adjust the pH to 5.5 with sodium oxide solution, stir for 15 minutes, add 30% sodium hydroxide solution dropwise to adjust the pH to 5.5, stir at room temperature for 2 hours, monitor the end of the reaction by HPLC, distill under re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com