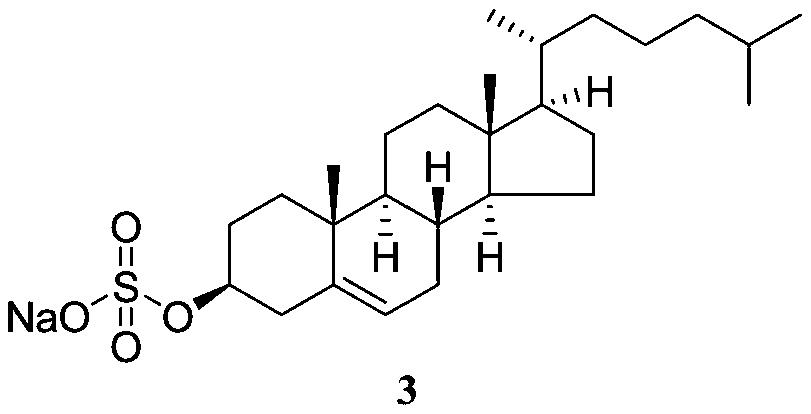
Preparation method of medicinal sodium cholesteryl sulfate
A cholesteryl sulfate, pharmaceutical grade technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, steroids, etc., can solve problems such as the exact yield of cholesteryl sulfate sodium, achieve low toxicity and ensure safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
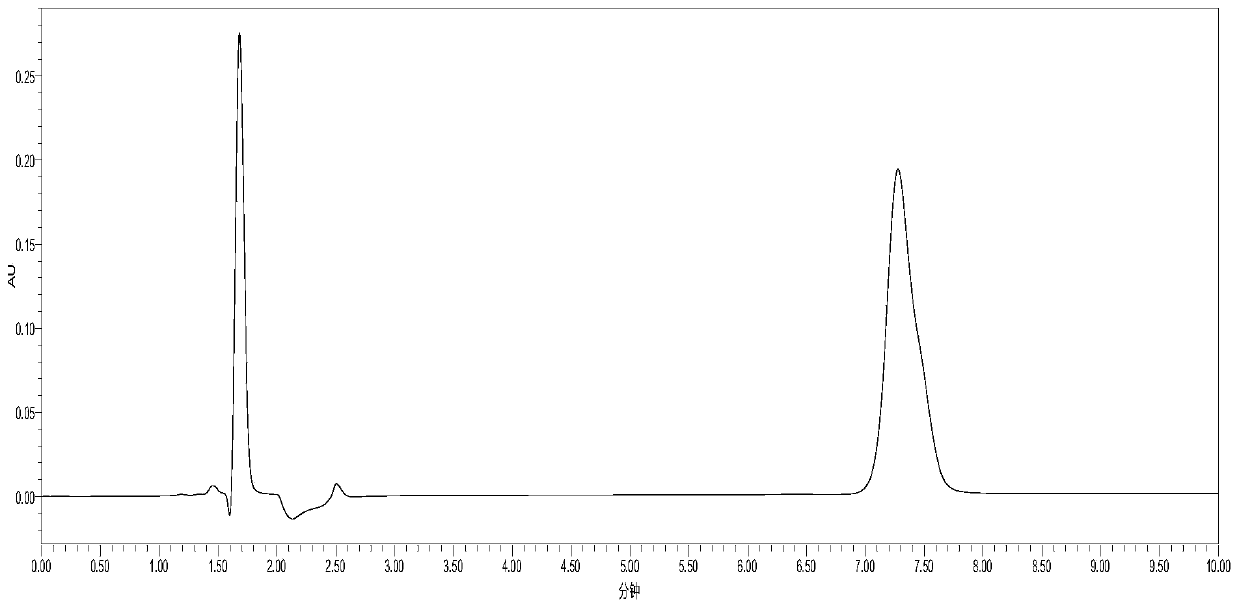
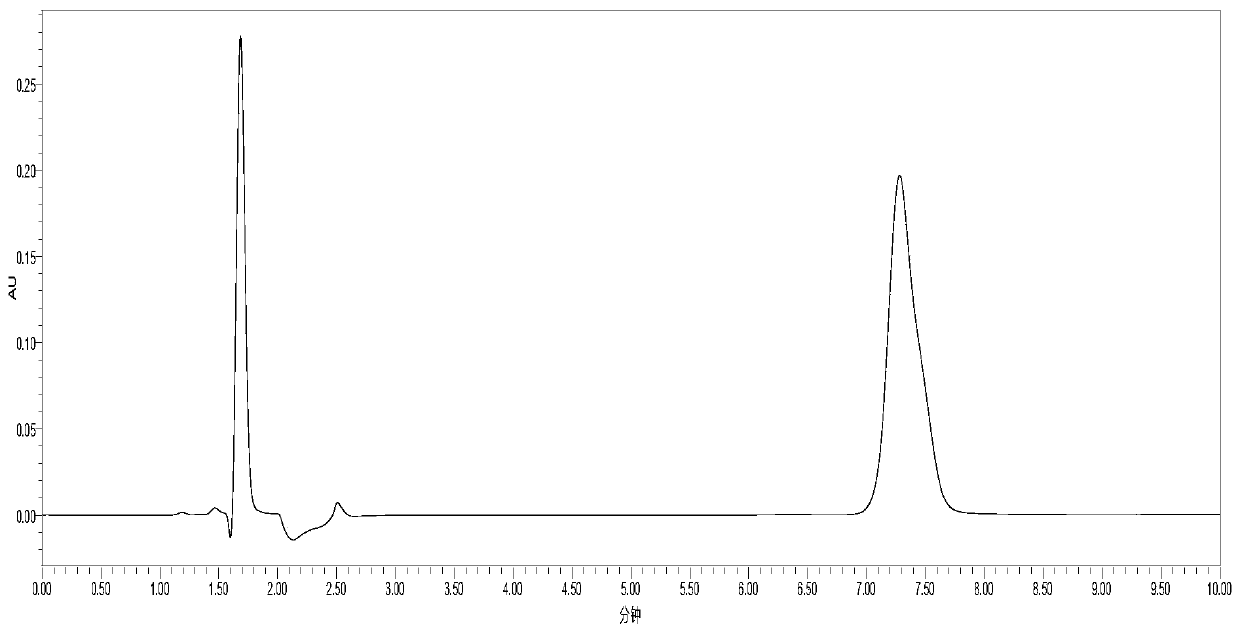
[0066] Experimental Example 1: HPLC Determination of Cholesterol Sulfate and Sodium Cholesterol Sulfate
[0067] Cholesterol sulfate and cholesterol are detected respectively by the HPLC method of Yang Zhimin et al. Sodium Sulfate:
[0068] Chromatographic conditions: liquid chromatography, UV detector, Kromasil C8 column (250mm×4.6mm, 5μm), 0.01mol / L ammonium acetate solution-acetonitrile (7:33) as mobile phase, detection wavelength 210nm.
[0069] Take 50mg of cholesteryl sulfate and sodium cholesteryl sulfate, weigh them accurately, place them in different 100mL measuring bottles, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution. Accurately measure 20 μL respectively and inject them into the liquid chromatograph, and record the chromatograms. in figure 1 for cholesterol sulfate, figure 2 Sodium Cholesterol Sulfate.
[0070] As can be seen from the figure, the peak times of cholesteryl sulfate and sodium cholesteryl sulfa...
Embodiment 1
[0080] Embodiment 1: the preparation of cholesterol sulfate
[0081]Put cholesterol and dimethylformamide (80mL) into a three-neck flask, heat and stir. After the cholesterol is completely dissolved, add sulfamic acid, heat to the reaction temperature, and stir vigorously for about 2 hours. After the reaction is complete, lower the temperature to 25°C and stir to crystallize until the crystallization is complete, filter, add anhydrous methanol to wash the filter cake, drain to obtain the intermediate product, and calculate the molar yield and residual cholesterol. When the above other conditions were the same, the amount of raw materials added and the reaction temperature were changed to observe their influence on the molar yield and residual cholesterol. The results are shown in Table 1.
[0082] Table 1. The impact of raw material addition and reaction temperature on the preparation of cholesteryl sulfate
[0083]
[0084] The results in Table 1 show that when the feed r...
Embodiment 2
[0100] Embodiment 2: the preparation of sodium cholesteryl sulfate
[0101] The intermediate product cholesterol sulfate (10 g cholesterol, 25.86 mmol) was prepared according to the method of Example 1-4.
[0102] The intermediate product cholesterol sulfate prepared in Examples 1-4 was added into anhydrous methanol, stirred and heated to the reaction temperature, and refluxed for 1 h. Add a solution of sodium hydroxide in anhydrous methanol (sodium hydroxide is dissolved in 30 mL of anhydrous methanol), control the temperature and continue to reflux for 3 hours, stir and cool down to 25°C, then stir and crystallize at 25°C, filter until no liquid flows out. Add purified water to wash the filter cake, suction filter until no liquid flows out, then wash the filter cake with anhydrous methanol, suction filter until no liquid flows out, and dry the filter cake to obtain the final product. Based on the amount of cholesterol fed, calculate the total molar yield . When above-menti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com