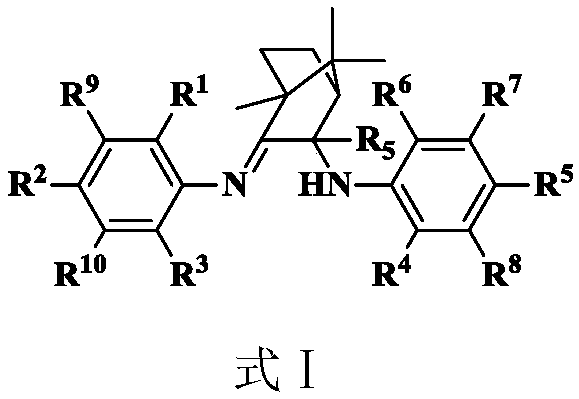
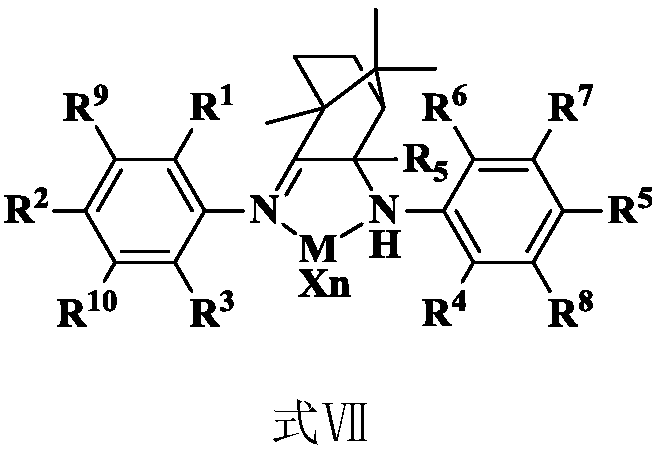
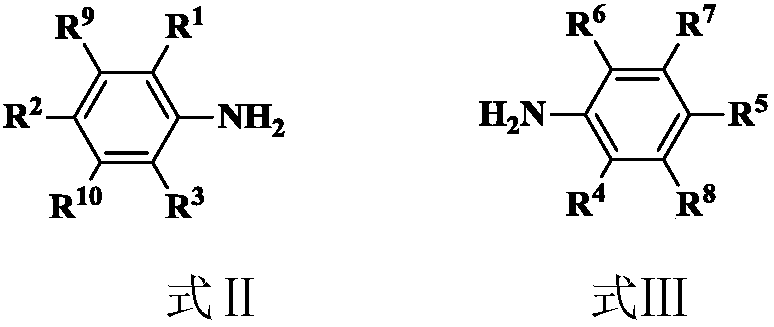Amino imine ligand, and amino imine coordination compound and applications thereof
An aminoimine and complex technology, which is applied in the field of aminoimine ligands, can solve the problems of low catalytic activity of aminoimine complexes, difficulty in designing and synthesizing ligand sterically hindered catalysts, etc., and achieves the effect of high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] 1) Preparation of ligand:
[0068] ɑ-diimine compound A1 3.88g (8mmol), add 30ml of toluene, 1M trimethylaluminum (16ml, 16mmol) in sequence, reflux for 8 hours, stop the reaction with sodium hydroxide / ice water, extract with ethyl acetate, and combine The organic phase was dried with anhydrous magnesium sulfate, and the product was separated by petroleum ether / ethyl acetate column chromatography to obtain colorless crystal ligand L1 with a yield of 84.2%. 1 HNMRδ(ppm) 7.19-7.06(m,6H,Ar-H),3.42(s,1H,NH), 2.98(m,2H,CH(CH 3 ) 2 ),2.88(m,2H,CH(CH 3 ) 2 ),2.32(m,1H,),1.81(m,4H,CH 2 ),1.50(s,3H,CH 3 ),1.21(m,24H,CH 3 ),0.92(s,3H,CH 3 ),0.75(s,3H,CH 3 ),0.72(s,3H,CH 3 ).
[0069] 2) Preparation of complex 1: add 10ml (DME) NiBr 2 (277mg, 0.9mmol) of dichloromethane solution was added dropwise to 10ml of ligand 1 (425mg, 0.9mmol) of dichloromethane solution, stirred at room temperature for 6 hours, precipitated out, filtered, washed with ether and dried to obtain a red powder solid....
Embodiment 2
[0072] 10atm ethylene polymerization: A 1L stainless steel polymerizer equipped with mechanical stirring was continuously dried at 130°C for 6hrs, vacuumed while hot, and N 2 Gas replacement 3 times. Add 7.2 mg (10 μmol) of complex 1 and then vacuum and replace with ethylene 3 times. Inject 500 ml of hexane, and then add 6.5 ml of methylaluminoxane (MAO) (1.53 mol / l toluene solution) to make Al / Ni=1000. At 60°C, maintaining an ethylene pressure of 10 atm, the reaction was stirred vigorously for 30 minutes. It is neutralized with an ethanol solution acidified with 5% hydrochloric acid to obtain polyethylene.
Embodiment 3
[0074] 10atm ethylene polymerization: A 1L stainless steel polymerizer equipped with mechanical stirring was continuously dried at 130°C for 6hrs, vacuumed while hot, and N 2 Gas replacement 3 times. Add 7.2 mg (10 μmol) of complex 1 and then vacuum and replace with ethylene 3 times. Inject 500 ml of hexane, and then add 6.5 ml of methylaluminoxane (MAO) (1.53 mol / l toluene solution) to make Al / Ni=1000. At 60° C., maintaining an ethylene pressure of 10 atm, vigorously stir the reaction for 10 min. It is neutralized with an ethanol solution acidified with 5% hydrochloric acid to obtain polyethylene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com