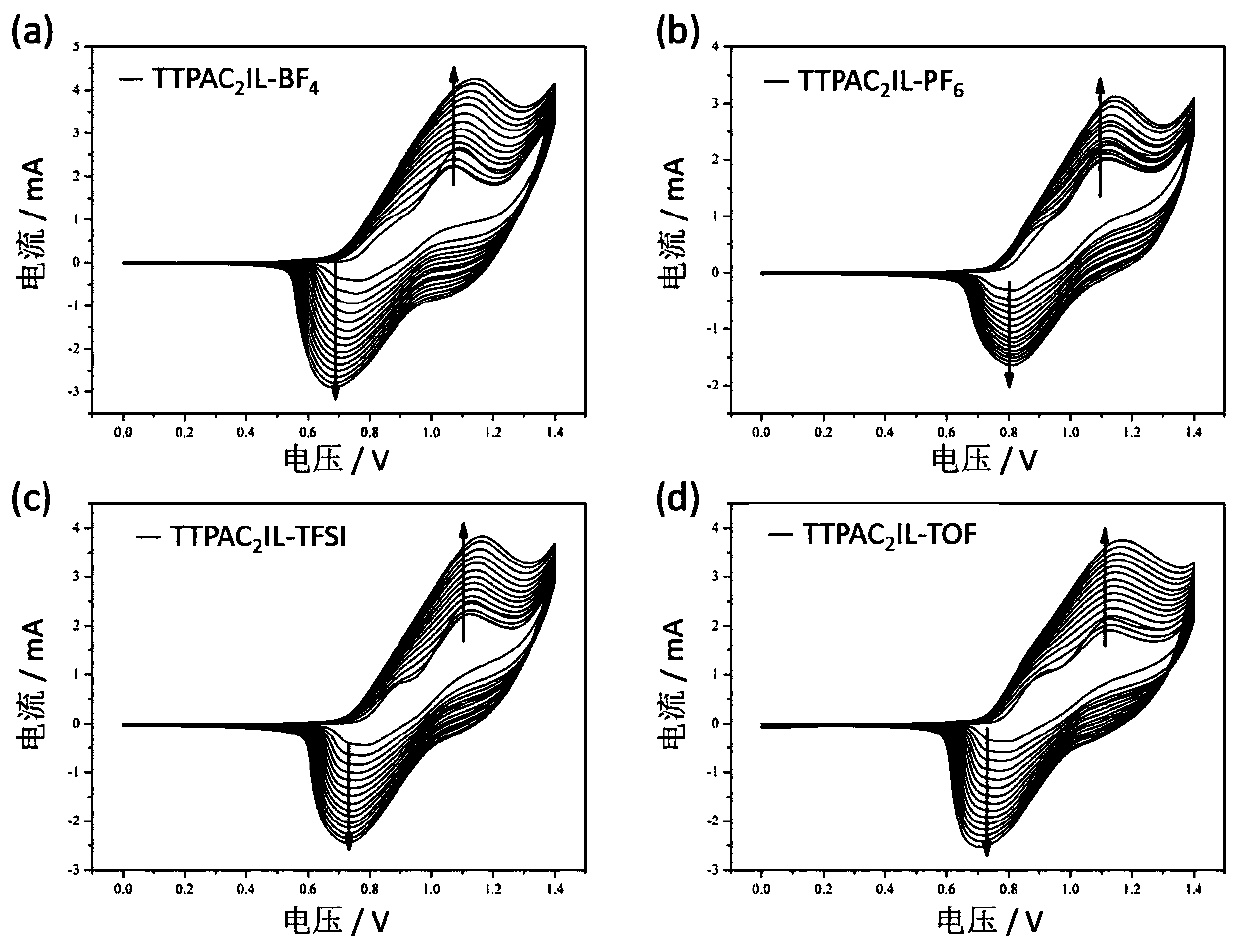
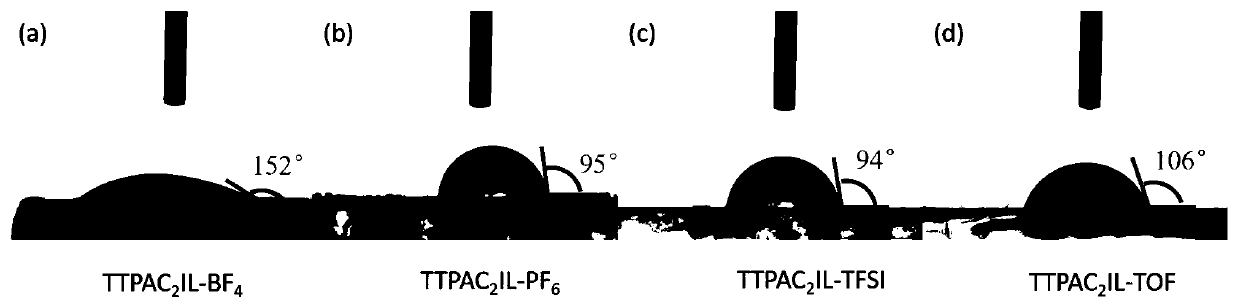
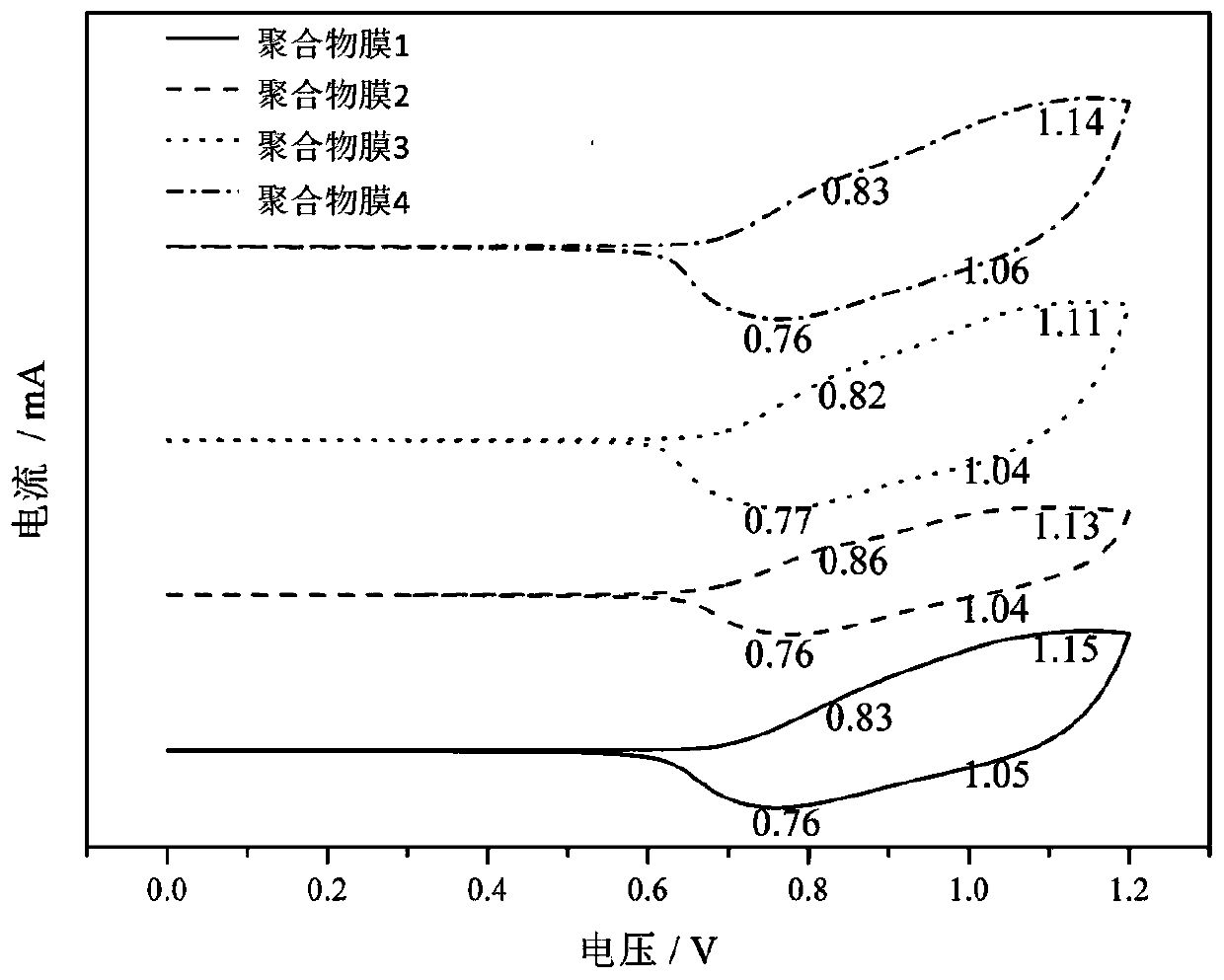
Ionic liquid containing triphenylamine structure as well as preparation method and application thereof
A technology of ionic liquid and triphenylamine, applied in chemical instruments and methods, color-changing fluorescent materials, organic chemistry, etc., can solve the problem of lack of systematic design of material structure and achieve the effect of reducing structural composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthesis of embodiment 1 4-(diphenylamino) benzonitrile
[0041]
[0042] Under nitrogen protection, sodium hydride (1.496 g, 62.3 mmol) was dissolved in DMF (40 mL), and stirred well until no bubbles were generated. Diphenylamine (5.11g, 30.2mmol) was added to the solution, and after the temperature rose to 110°C and stabilized, p-fluorobenzonitrile (4.49g, 37.1mmol) was slowly added, and the system was refluxed for 12h. After the reaction was completed, the reaction liquid was cooled to room temperature, and saturated brine and dichloromethane were added for extraction, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Using 300-400 mesh silica gel as the stationary phase, eluting with dichloromethane / petroleum ether mixed solution with a volume ratio of 1:1 as the mobile phase, collecting the eluate containing the target compound, distilling of...
Embodiment 2
[0043] Example 2 Synthesis of 4-(bis(4-bromophenyl)amino)benzonitrile
[0044]
[0045] Under dark conditions, the intermediate product 4 (dianilino) benzonitrile compound (4 g, 14.8 mmol) was dissolved in DMF (30 mL), and NBS (6.59 g, 37.1 g) dissolved in DMF (15 mL) was added dropwise. mmol) solution, reacted at room temperature for 24h. After the reaction was completed, the reaction liquid was cooled to room temperature, and saturated brine and dichloromethane were added for extraction, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Using 300-400 mesh silica gel as the stationary phase, eluting with dichloromethane / petroleum ether mixed solution with a volume ratio of 1:1 as the mobile phase, collecting the eluate containing the target compound, distilling off the solvent under reduced pressure and drying to obtain the following: 6.01 g of the white po...
Embodiment 3
[0046] Example 3 Synthesis of 4-(bis(4-(thiophene-2)phenyl)amino)benzonitrile
[0047]
[0048] Under nitrogen protection, the intermediate product 4-(bis(4-bromophenyl)amino)benzonitrile (3.75g, 8.76mmol), potassium carbonate (5.4g, 39.12mmol), bis(triphenylphosphine) di Palladium chloride (780mg, 1.11mmol) and 2-thiopheneboronic acid (3.9g, 30.47mmol) were dissolved in a mixed solution of methanol (50mL) and toluene (50mL), and reacted at 100°C for 24h. After the reaction was completed, the reaction liquid was cooled to room temperature, and saturated brine and dichloromethane were added for extraction, and the organic phase was collected, dried over anhydrous magnesium sulfate, concentrated under reduced pressure, and separated and purified by column chromatography. Using 300-400 mesh silica gel as the stationary phase, eluting with dichloromethane / petroleum ether mixed solution with a volume ratio of 1:1 as the mobile phase, collecting the eluate containing the target c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com