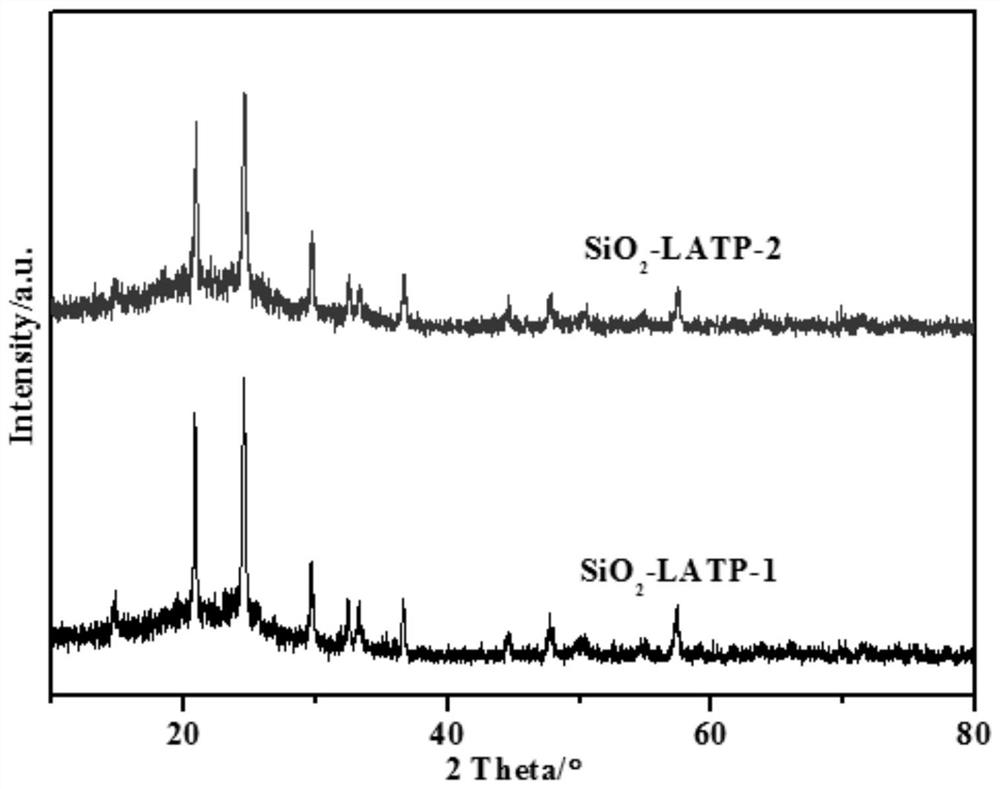
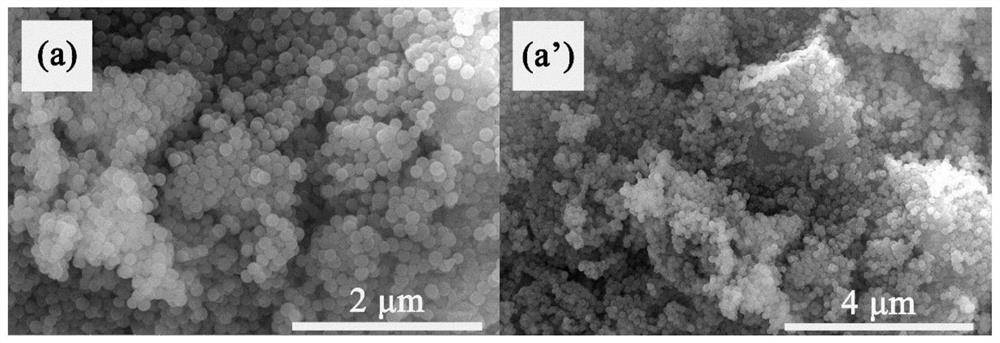
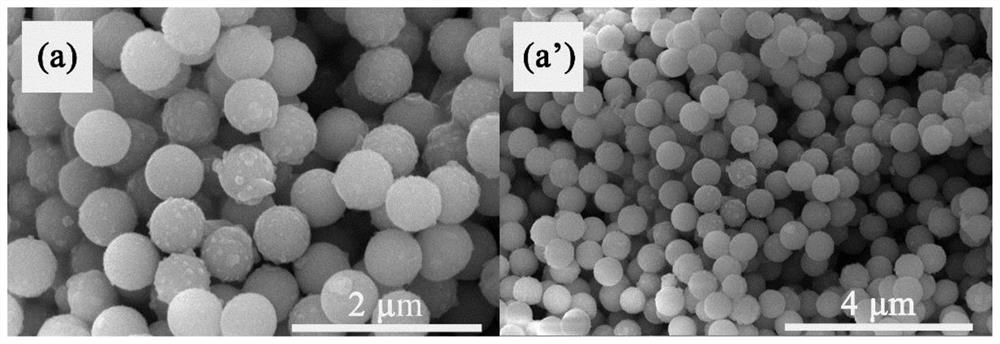
A core-shell structure inorganic composite material and its preparation and application
An inorganic composite material, core-shell structure technology, applied in nanotechnology, nanotechnology, electrical components and other directions for materials and surface science, can solve the problems of low conductivity of PEO-based polymer electrolytes, and achieve reduced crystallinity, The effect of accelerating the ion conduction rate and expanding the area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Take a 250mL Erlenmeyer flask, add 100mL of absolute ethanol and 4.16g of ethyl orthosilicate to it, and stir magnetically to form solution 1; take another 250mL Erlenmeyer flask, add 80mL of anhydrous Ethanol, 5.28g of deionized water and 7.7mL of concentrated ammonia water were magnetically stirred to form solution 2; solution 2 was slowly added to solution 1, magnetically stirred at 2000 rpm for 24 hours, and then stirred at 10000 rpm The white precipitate was separated by centrifugation, washed three times with absolute ethanol, and the obtained precipitate was dried at 60°C for 12 hours to obtain spherical SiO 2 Material.
[0039] Weigh 0.4g of SiO 2 materials, were added to 40mL of absolute ethanol, ultrasonicated for 30min to form a uniform suspension, and then 0.0441g LiNO was added to the solution 3 , 0.0503g Al(NO 3 ) 3 9H 2 O, 0.2586g tetrabutyl titanate and 0.1546g phosphoric acid, magnetically stirred to dissolve completely, then added 0.4481g EDTA and...
Embodiment 2
[0042] Take a 250mL Erlenmeyer flask, add 50mL of absolute ethanol and 4.16g of tetraethyl orthosilicate to it, and stir magnetically to form solution 1; take another 250mL Erlenmeyer flask, add 40mL of anhydrous Ethanol, 5.64g deionized water and 7.7mL concentrated ammonia water were magnetically stirred to form solution 2; solution 2 was slowly added to solution 1, magnetically stirred at 2000 rpm for 24 hours, and then stirred at 10000 rpm The white precipitate was separated by centrifugation, washed three times with absolute ethanol, and the obtained precipitate was dried at 60°C for 12 hours to obtain spherical SiO 2 Material.
[0043] Weigh 0.4g of SiO 2 materials, were added to 40mL of absolute ethanol, ultrasonicated for 30min to form a uniform suspension, and then 0.0441g LiNO was added to the solution 3 , 0.0503g Al(NO 3 ) 3 9H 2 O, 0.2586g tetrabutyl titanate and 0.1546g phosphoric acid, magnetically stirred to dissolve completely, then added 0.4481g EDTA and 0...
Embodiment 3
[0046] Add 0.6g of PEO and 0.2175g of LITFSI to 6mL of anhydrous acetonitrile solvent, stir magnetically, stir for 12h, let stand for 2h to form a uniform electrolyte slurry, pour the slurry into a polytetrafluoroethylene mold, and use The stainless steel blade was scraped flat, transferred to a vacuum drying oven, dried at 50°C for 24 hours, and cooled to room temperature to form a PEO / LITFSI solid lithium ion electrolyte membrane. The conductivity of the composite electrolyte was studied by AC impedance technology, and the specific measurement conditions were as follows: in an open circuit state, the test temperature was 25-95°C, and the frequency range was 10 6 Hz-1Hz, the interference voltage is 5mV. see test results Figure 6 , as the test temperature increases, the total resistance of the electrolyte membrane decreases gradually, because the movement of ions and polymer chains inside the electrolyte membrane accelerates. According to the conductivity calculation formula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com