Methane cracking catalyst and preparation method thereof
A technology for cracking catalysts and catalysts, which is applied in the directions of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., which can solve problems such as increasing reaction temperature and easy deactivation of Ni catalysts, and achieve uniform and stable composition. The effect of high tone and dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
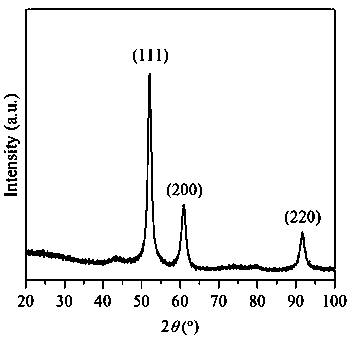
Embodiment 1
[0030] Weigh 20 g of NaOH solid, dissolve in 250 mL of deionized water, stir for 10 min, and make 2 mol / L NaOH aqueous solution. Moore (Ni 2+ +Cu 2+ ):Al 3+ = 75:25, Ni 2+ :Cu 2+ = 90:10, weigh 7.8513g Ni (NO 3 ) 2 ·6H 2 O, 0.7248g Cu(NO 3 ) 2 ·3H 2 O, 3.7513g Al(NO 3 ) 3 9H 2 O was dissolved in 100 mL deionized water and stirred for 10 min to completely dissolve the nitrate to obtain a mixed solution. Press Na 2 CO 3 The molar mass is Al(NO 3 ) 3 9H 2 Half of the molar weight of O weighs 0.5300gNa 2 CO 3 , dissolved in 100 mL deionized water as the base solution. Use the dropping funnel to drop the nickel-copper-aluminum nitrate mixed solution into the solution containing Na 2 CO 3 solution in a beaker with constant stirring. At the same time, use a peristaltic pump to slowly drop the precipitant NaOH solution into the beaker at a rate of 35 drops / min, maintain the precipitation pH = 10 ± 0.5, continue to stir for 1 h after the addition, then let it s...
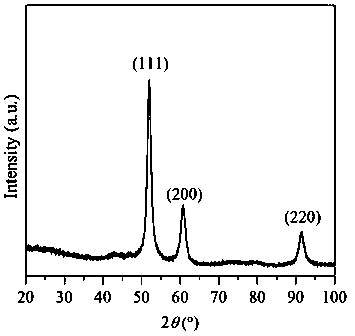
Embodiment 2
[0033] Weigh 20 g of NaOH solid, dissolve in 250 mL of deionized water, stir for 10 min, and make 2 mol / L NaOH aqueous solution. Moore (Ni 2+ +Cu 2+ ):Al 3+ = 75:25, Ni 2+ :Cu 2+ = 80:20, weigh 6.9790g Ni (NO 3 ) 2 ·6H 2 O, 1.4496g Cu(NO 3 ) 2 ·3H 2 O, 3.7513g Al(NO 3 ) 3 9H 2 O was dissolved in 100 mL deionized water and stirred for 10 min to completely dissolve the nitrate to obtain a mixed solution. Press Na 2 CO 3 The molar mass is Al(NO 3 ) 3 9H 2 Half of the molar weight of O weighs 0.5300gNa 2 CO 3 , dissolved in 100 mL deionized water as the base solution. Use the dropping funnel to drop the nickel-copper-aluminum nitrate mixed solution into the solution containing Na 2 CO 3 solution in a beaker with constant stirring. At the same time, use a peristaltic pump to slowly drop the precipitant NaOH solution into the beaker at a rate of 35 drops / min, maintain the precipitation pH = 10 ± 0.5, continue to stir for 1 h after the addition, then let it st...
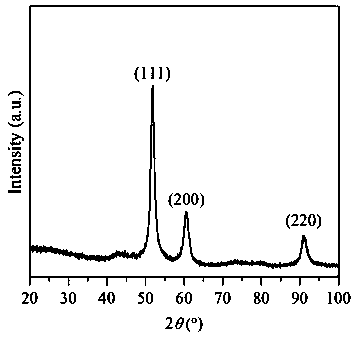
Embodiment 3
[0036] Weigh 20 g of NaOH solid, dissolve in 250 mL of deionized water, stir for 10 min, and make 2 mol / L NaOH aqueous solution. Moore (Ni 2+ +Cu 2+ ):Al 3+ = 75:25, Ni 2+ :Cu 2+ = 70:30, weigh 6.1066g Ni (NO 3 ) 2 ·6H 2 O, 2.1744g Cu(NO 3 ) 2 ·3H 2 O, 3.7513g Al(NO 3 ) 3 9H 2 O was dissolved in 100 mL deionized water and stirred for 10 min to completely dissolve the nitrate to obtain a mixed solution. Press Na 2 CO 3 The molar mass is Al(NO 3 ) 3 9H 2 Half of the molar weight of O weighs 0.5300gNa 2 CO 3 , dissolved in 100 mL deionized water as the base solution. Use the dropping funnel to drop the nickel-copper-aluminum nitrate mixed solution into the solution containing Na 2 CO 3 solution in a beaker with constant stirring. At the same time, use a peristaltic pump to slowly drop the precipitant NaOH solution into the beaker at a rate of 35 drops / min, maintain the precipitation pH = 10 ± 0.5, continue to stir for 1 h after the addition, then let it s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com