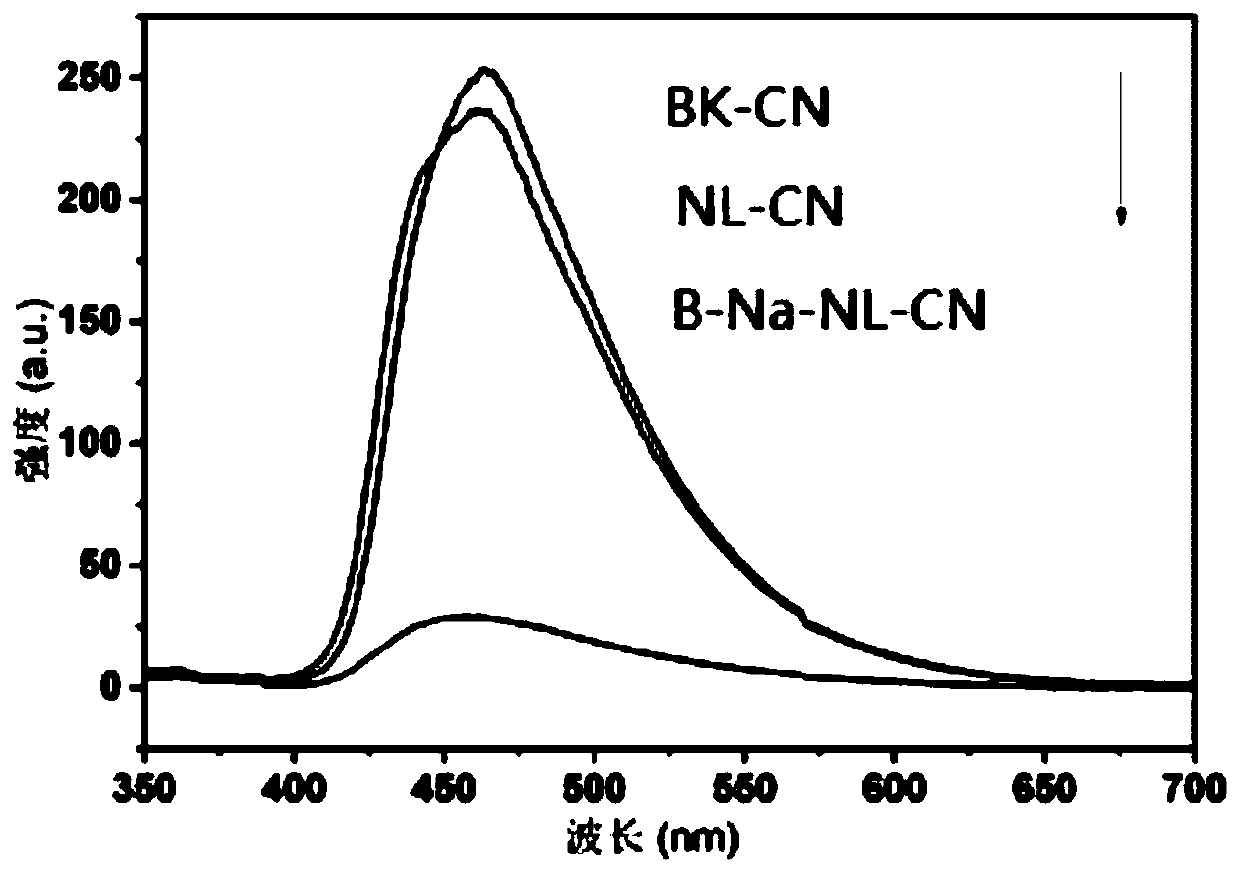
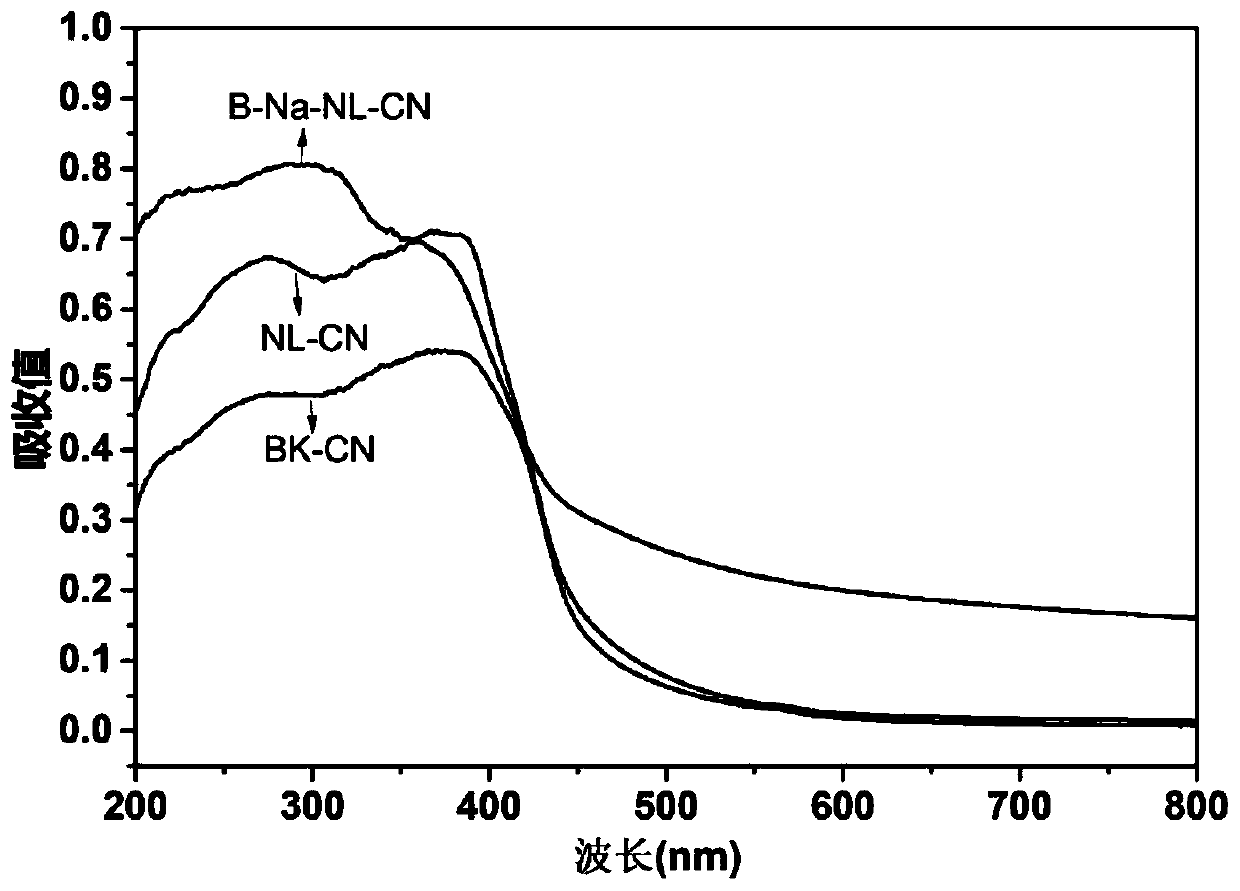
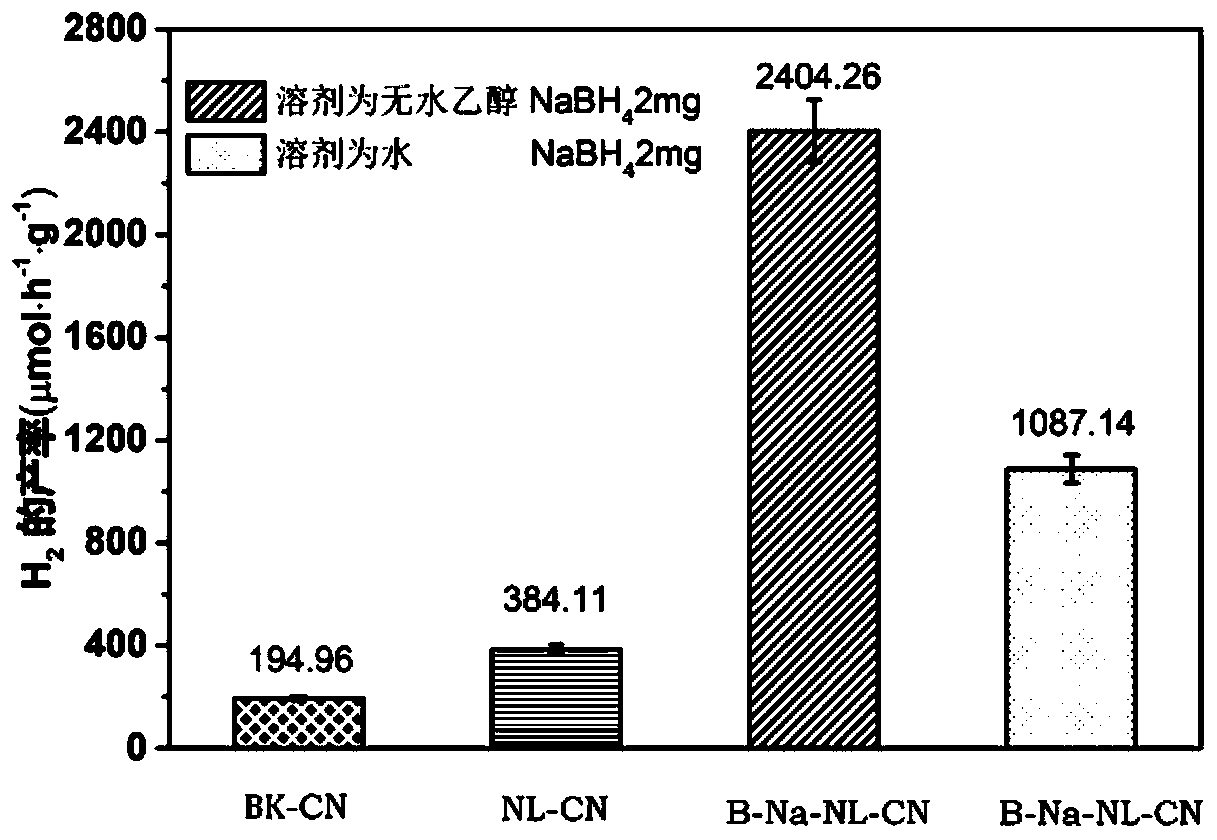
Boron-sodium double-doped nano-layered graphite-like carbon nitride, and preparation method and application thererof
A graphite-phase carbon nitride and nano-layered technology, applied in chemical instruments and methods, inorganic chemistry, chemical/physical processes, etc., can solve the problems of small specific surface area, high photogenerated electron-hole recombination rate, and low photocatalytic efficiency To achieve the effects of enhancing light absorption, improving hydrogen production efficiency, and reducing recombination rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The invention provides a method for preparing boron-sodium double-doped nano-layer graphite-like carbon nitride, the preparation method comprising the following steps:
[0046] S1, dissolving melamine in water to obtain a melamine solution, adding ammonium nitrate to the melamine solution, after the reaction is completed, calcining the obtained solid product to obtain a nano-layered structure g-C 3 N 4 ;
[0047] S2, the nano-layered structure g-C 3 N 4 with NaBH 4 The reaction is mixed and the reaction product is calcined and cooled to obtain a boron-sodium double-doped nano-layered structure g-C 3 N 4 .
[0048] As an embodiment, in the step S1, the molar ratio of ammonium nitrate to melamine is 2 to 5 (for example, the molar ratio of ammonium nitrate to melamine can be specifically 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7, 2.8, 2.9, 3, 3.1, 3.2, 3.3, 3.4, 3.5, 3.6, 3.7, 3.8, 3.9, 4, 4.1, 4.2, 4.3, 4.4, 4.5, 4.6, 4.7, 4.8, 4.9 or 5).
[0049] When the molar ratio of ...
Embodiment 1
[0076] A method for preparing boron-sodium double-doped nano-layer graphite-like carbon nitride, comprising the following steps:
[0077] S1: nano-layered structure g-C 3 N 4 preparation of
[0078] S11, 7.5672g (60mmol) of melamine was dissolved in 300mL of water, and heated to 95°C to obtain a melamine solution.
[0079] S12, under stirring conditions, dissolve 9.6g (120mmol) of ammonium nitrate in 20ml of water, and add dropwise to the melamine solution at a rate of 1-3 seconds per drop. After the dropwise addition, react for 1 hour.
[0080] S13, after the reaction is completed, cool to room temperature to obtain a white floc, filter the precipitate to obtain melamine ammonium dinitrate, place the melamine ammonium dinitrate in a drying oven at 60° C., and dry for 12 hours.
[0081] S14, put the dried melamine ammonium dinitrate into a 50mL crucible and cover it, place it in a muffle furnace for calcination at 350°C for 2 hours, and the heating rate is 1°C / min; then at ...
Embodiment 2
[0088] The difference between this example and Example 1 is that the amount of sodium borohydride in step S21 is 5 mg, and other steps and process parameters are consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com