Preparation of magnetic core-shell conductive polymer loaded nanogold catalyst and application of magnetic core-shell conductive polymer loaded nanogold catalyst in p-nitrophenol hydrogenation
A conductive polymer, core-shell catalyst technology, applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, preparation of aminohydroxy compounds, etc., can solve the problem of poor stability of nano-gold catalysts, difficult to recycle, etc problem, to achieve the effect of excellent catalytic activity, easy separation and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: A magnetic Au@Fe 3 o 4 Preparation method of conductive polymer core-shell catalyst and its application in catalytic selective hydrogenation of p-nitrophenol
[0031] 1.8g FeCl 3 ·6H 2O was dissolved in 150ml of ethylene glycol and dissolved under magnetic stirring; 1.98g of sodium acetate was weighed and added to the above system, and magnetic stirring was continued until a homogeneous solution was obtained, then the solution was put into a hydrothermal synthesis kettle for 7 hours at 110°C. The resulting precipitate was subjected to magnetic separation, washed repeatedly with deionized water and absolute ethanol until the filtrate was colorless, and dried at 50°C under vacuum to constant weight (about 8 hours) to obtain a solid powder.
[0032] Grind the above solid powder and weigh 0.5g, add it to a mixed solution containing 100 ml hydrochloric acid (15 mmol / L) and 0.7 ml aniline monomer, then add 11 ml ammonium persulfate solution dropwise under mechan...
Embodiment 2
[0036] Example 2: A magnetic Au@Fe 3 o 4 Preparation method of conductive polymer core-shell catalyst and its application in catalytic selective hydrogenation of p-nitrophenol
[0037] The specific experimental steps are the same as in Example 1, only changing Au@Fe 3 o 4 The gold loading method of the conductive polymer core-shell catalyst, the gold precursor is changed into a chloroauric acid solution, and the gold is loaded using the conductive polymer in situ reduction method to obtain 0.1g Fe 3 o 4 Add 0.052ml of chloroauric acid solution (the concentration of chloroauric acid solution is 9.56mg / ml) and 10ml of deionized water to the conductive polymer core-shell carrier. Au@Fe was obtained after overnight stirring, magnetic separation, washing and drying 3 o 4 / Conductive polymer core-shell catalysts.
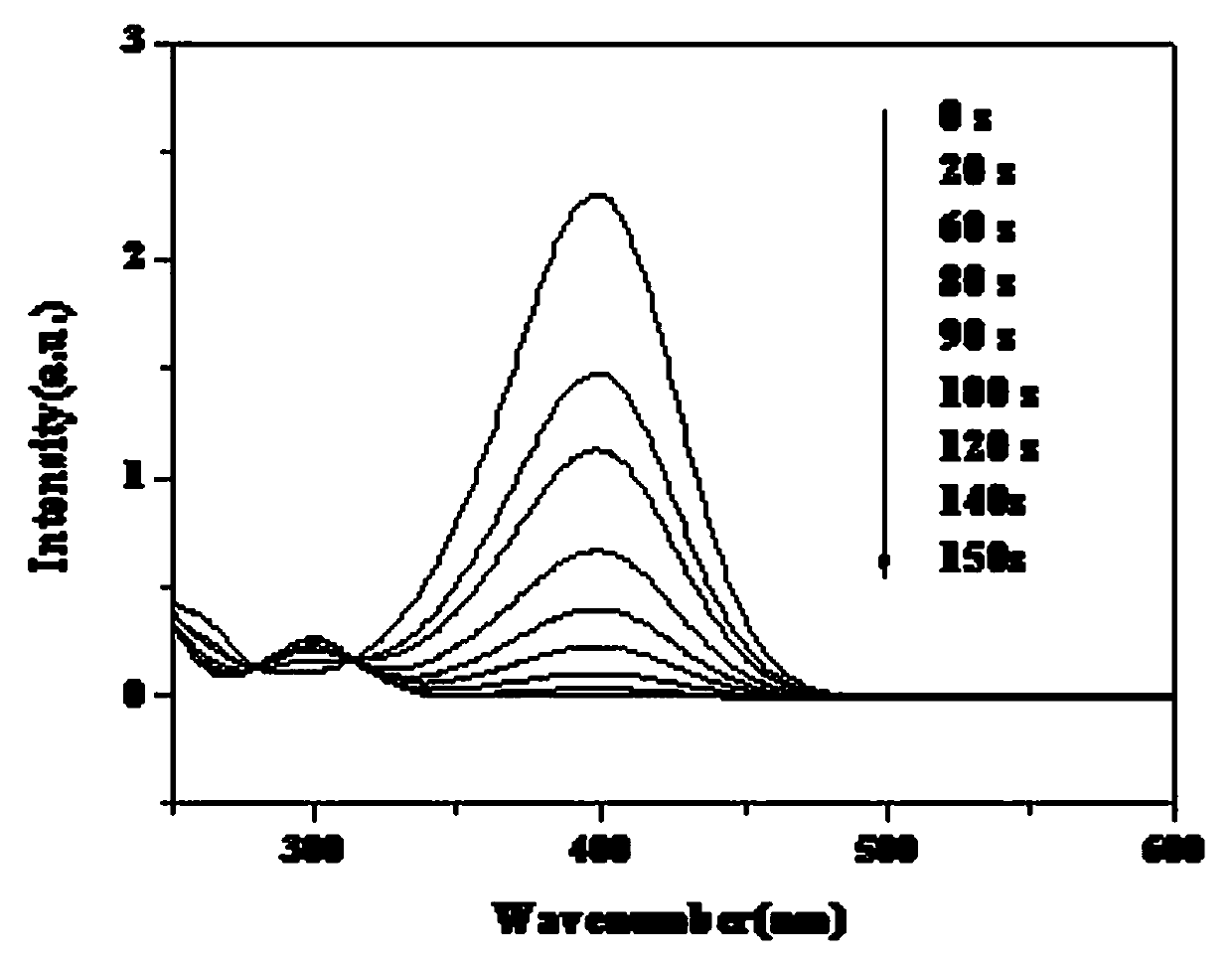
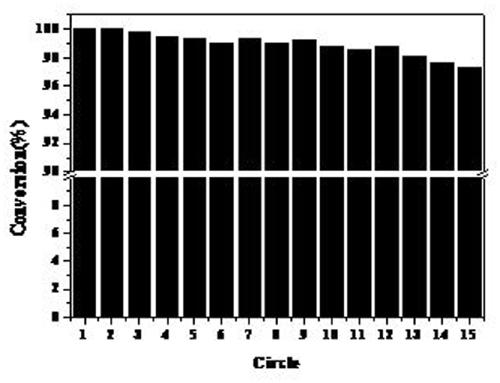
[0038] image 3 The catalyst prepared under the conditions of the examples is an activity diagram of the obtained circulating catalytic reaction product under th...
Embodiment 3
[0039] Example 3: A magnetic Au@Fe 3 o 4 Preparation method of conductive polymer core-shell catalyst and its application in catalytic selective hydrogenation of p-nitrophenol
[0040] 5.4g FeCl 3 ·6H 2 O was dissolved in 180ml of ethylene glycol and dissolved under magnetic stirring; 16.2g of sodium acetate was weighed and added to the above system, and magnetic stirring was continued until a uniform solution was obtained, then the solution was put into a hydrothermal synthesis kettle at 300°C for 10 hours. The obtained precipitate was subjected to magnetic separation, washed repeatedly with deionized water and absolute ethanol until the filtrate was colorless, and dried at 50°C under vacuum to constant weight (about 12h) to obtain a solid powder.
[0041] Grind the above solid powder and weigh 0.5g, add it to the mixed solution containing 100 ml hydrochloric acid (10 mmol / L) and 0.4 ml pyrrole monomer, then add 8.5 ml ammonium persulfate solution dropwise under mechanical...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com