NADH analog dependent cytochrome P450 reductase and application thereof
A technology of cytochromes and analogs, applied in the direction of oxidoreductases, enzymes, enzymes, etc., can solve the problems of weak host substrate supply capacity, restrictions on industrial applications, and low overall efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
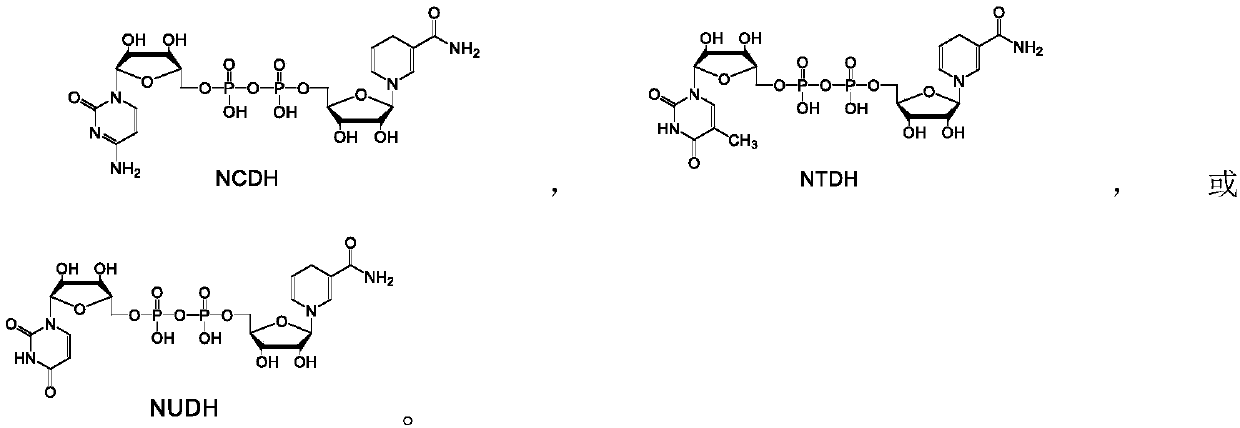
[0036] Example 1: Enzymatic reduction of NAD analogs NCD, NTD and NUD to reduced NADH analogs NCDH, NTDH and NUDH.
[0037] The NAD analogs NCD, NTD and NUD were reduced with malic enzyme ME-L310R / Q401C, D-lactate dehydrogenase DLDH-V152R, phosphorous acid dehydrogenase PDH-I151R or formate dehydrogenase FDH-G171Y, respectively, according to The following method was used for the reaction: 1 mM of NAD analogs, 4 mM of substrate (one of malic acid, D-lactic acid, phosphorous acid or formic acid) and 10 U of enzyme (malic enzyme ME-L310R / Q401C, D- One of lactate dehydrogenase DLDH-V152R, phosphite dehydrogenase PDH-I151R or formate dehydrogenase FDH-G171Y) was dissolved in 1 mL of HEPES buffer with a concentration of 50 mM and pH 7.5, and reacted at 30°C for 20 min. Take 20 μL for analysis.
[0038] Analysis found that all samples had characteristic absorption peaks at 340nm, indicating that malic enzyme ME-L310R / Q401C, D-lactate dehydrogenase DLDH-V152R, phosphorous acid dehydr...
Embodiment 2
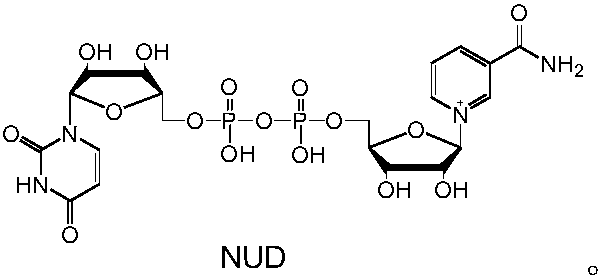
[0041] Example 2: Enzymatic preparation of reduced NAD analogs
[0042] The reaction system in Example 1 is scaled up and can be used to prepare reduced NAD analogs. Taking formate dehydrogenase FDH-G171Y to prepare NUDH from sodium formate as an example, the preparation process is illustrated. 20 mM NUD, 25 mM sodium formate and 5 mg of formate dehydrogenase FDH-G171Y were dissolved in 10 mL of sodium phosphate buffer solution with a concentration of 50 mM and a pH of 7.5, mixed well, and reacted at 30°C for 2 h. Freeze-dry directly after the reaction, concentrate to a total volume of about 4mL, separate with a formic acid-type anion-exchange resin column (201×4), track and collect the product at an ultraviolet wavelength of 340nm, and freeze-dry to obtain 11.6mg of white powder with a yield of about 90 %. The above-mentioned white powder sample was subjected to high-resolution mass spectrometry analysis to measure the precise molecular weight (M+H) + It is 643.1026, and t...
Embodiment 3
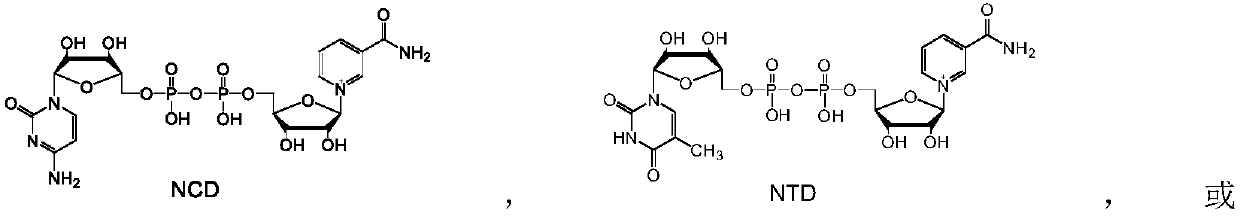
[0044] Example 3: Chemical reduction of NAD analogues NCD, NTD and NUD to reduced NADH analogues NCDH, NTDH and NUDH.
[0045] The NAD analogs NCD, NTD and NUD, respectively, with Na 2 S 2 o 4 , NaBH 4 、NaBH(Et) 3 , NaBH 3 For CN reduction, the reaction was carried out as follows: 1 mM NAD analogue, 4 mM reducing agent (Na 2 S 2 o 4 , NaBH 4 、NaBH(Et) 3 , NaBH 3 CN) dissolved in 1 mL of H 2 Mix well in O, react at 30°C for 20 min, and take 20 μL for analysis.
[0046] The analysis found that all samples had characteristic absorption peaks at 340nm, indicating that Na 2 S 2 o 4 , NaBH 4 、NaBH(Et) 3 , NaBH 3 CN can reduce NAD analogs. The quantitative method is the same as in Example 1, and the results are shown in Table 2. Visible chemical reducing agent Na 2 S 2 o 4 , NaBH 4 、NaBH(Et) 3 , NaBH 3 CN can be used to reduce NAD analogs to prepare NADH analogs without substrate selectivity.
[0047] Table 2 Experimental results of reducing NAD analogues by...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com