Streptodornase B antigen and application thereof
An antigen and nucleic acid sequence technology, applied in the field of genetic engineering, can solve problems such as deviation of detection results, complicated operation, and low yield, and achieve the effects of no safety risk, simple preparation method, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of the DNaseB fusion dominant epitope antigen of the present application
[0033] 1. Amino acid sequence and coding sequence of DNase B fusion dominant epitope antigen
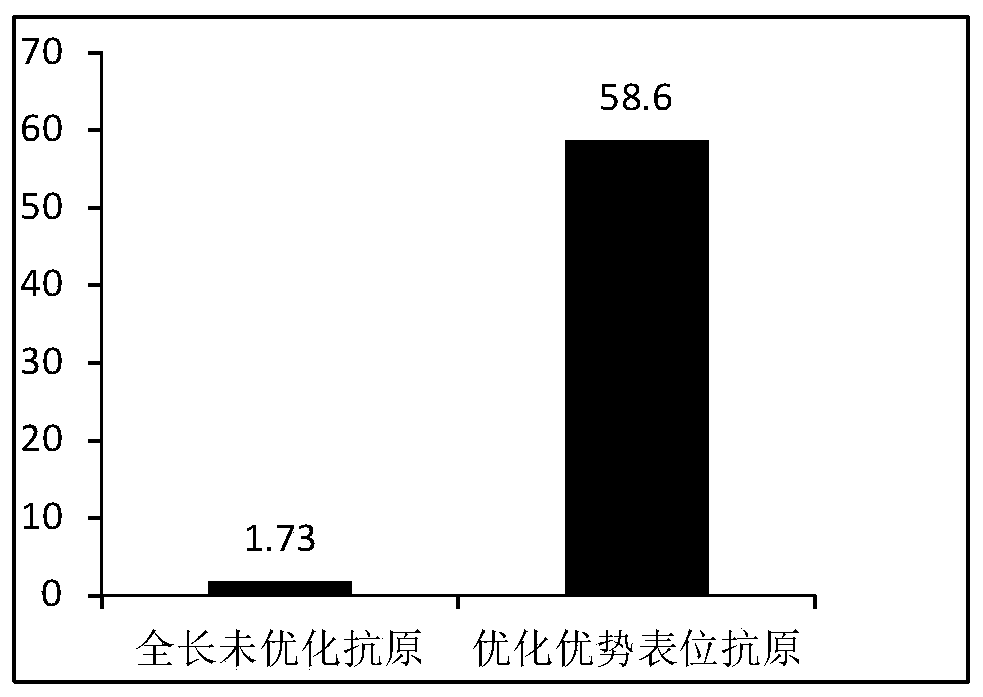
[0034] The original DNaseB (streptococcal DNase B) in Genebank has a full length of 271 amino acids, contains a 43aa leader peptide and 228 amino acids of a mature protein, and the sequence of the mature protein is shown in SEQ ID NO.3 in the sequence table, with a molecular weight of about It is 25.4kD. The DNaseB antigen of the present application (also known as DNase B fusion-dominant epitope antigen, which is different from the original DNaseB) has undergone gene optimization and epitope screening, and its amino acid sequence is shown in SEQ ID NO.1 in the sequence listing. The optimized gene sequence encoding DNaseB antigen is shown as SEQ ID NO.2 in the sequence listing.
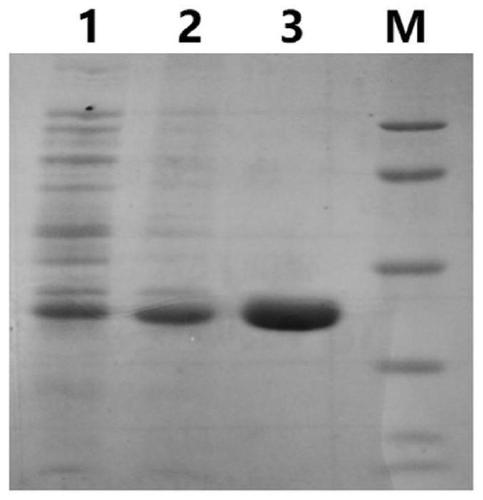
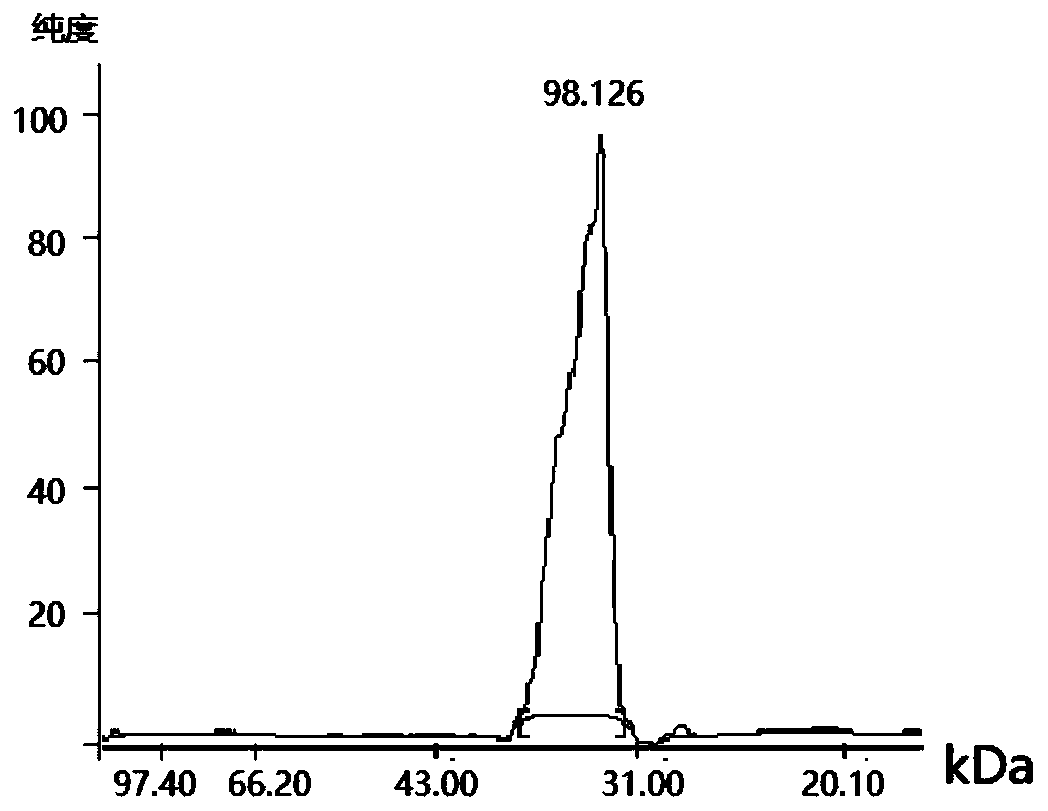
[0035] 2. Expression and purification of DNaseB genetic engineering fusion antigen
[0036] According...
Embodiment 2
[0040] Example 2 Activity Identification of DNaseB Genetic Engineering Fusion Antigen
[0041] The DNase B fusion-dominant epitope antigen obtained after purification was routinely subjected to 10% SDS-PAGE electrophoresis, transferred to a membrane, and blocked with 5% skimmed milk powder at room temperature for 1 h. Specific anti-DNase B antibody and non-specific antibody (anti-actin antibody) diluted in blocking solution were incubated overnight at 4°C. After washing the membrane three times at room temperature with TBST, the membrane was incubated with horseradish peroxidase-labeled secondary antibody for 1 h. The membrane was washed three times with TBST at room temperature, and the positive bands were displayed by ECL method, scanned and photographed. The result is as Figure 4 As shown, the obtained DNase B fusion-dominant epitope antigen can be well recognized by specific anti-DNase B antibodies, but not by non-specific antibodies, indicating that the obtained DNase ...
Embodiment 3
[0042] Example 3 Establishment of Anti-DNaseB Antibody Double Antigen Sandwich Detection Kit
[0043] 1. Anti-DNaseB Antibody Double Antigen Sandwich Detection Kit
[0044] Anti-DNaseB Antibody Double Antigen Sandwich Detection Kit mainly includes:
[0045] ELISA plate coated with DNase B fusion-dominant epitope antigen, horseradish peroxidase (HRP)-labeled DNase B fusion-dominant epitope antigen, sample diluent, washing solution, TMB chromogenic substrate A solution, TMB chromogenic substrate Color substrate B solution and reaction termination solution.
[0046] Sample diluent: 1% BSA, 5mM EDTA, 5% goat serum, 0.1% Proclin-300, 0.1% Triton-X100, 1.78% NaCl, 0.04% Thimerosal.
[0047] Washing solution: 5.63% Na 2 HPO 4 12H 2 O, 0.672% NaH 2 PO 4 2H 2 O, 17% NaCl, 1% Tween-20.
[0048] TMB chromogenic substrate A solution: 0.32% citric acid (monohydrate), 0.06% hydrogen peroxide solution, 2.72% NaAc·3H 2 o
[0049] TMB chromogenic substrate solution B: 0.04% EDTA-Na ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com