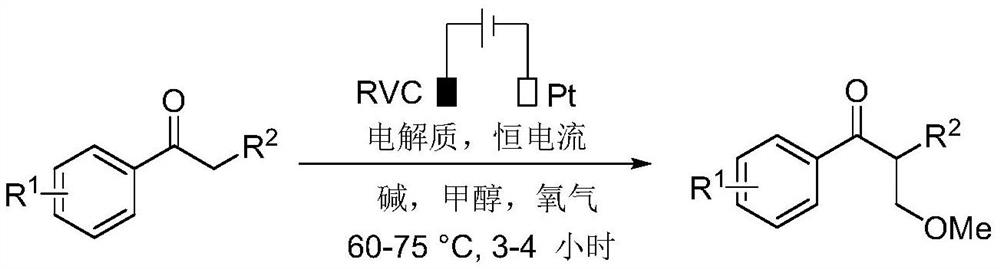
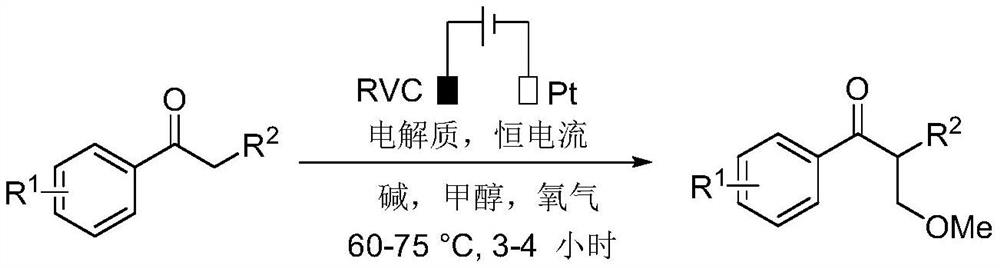
A kind of method for realizing methylation reaction of ketone α position under electrochemical conditions
A methylation and electrochemical technology is applied in the field of realizing the methylation reaction at the α position of ketone, and the effect of cheap and easy to obtain reaction raw materials, high yield, and simple atomic economy is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
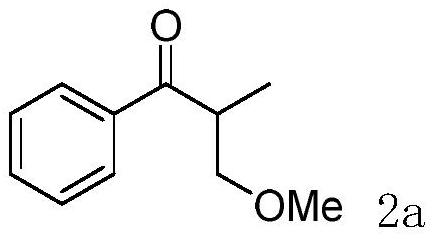
[0016] Preparation and characterization of 2-methyl-3-methoxy-1-phenyl-1-propanone (2a):
[0017]
[0018] Place propiophenone (0.5mmol, 1.0equiv), cesium carbonate (1.0mmol, 2equiv) and tetrabutylammonium tetrafluoroborate (0.25mmol, 0.5equiv) in a 10mL three-neck round bottom flask equipped with a condenser , RVC (100ppi, 1cmx1cmx1.2cm) anode and platinum plate (1cmx1cm) cathode, put on an oxygen bulb and add 6mL methanol to the reaction bottle, and react at 65°C with a constant current of 10mA until the raw materials are completely consumed (monitored by thin-layer chromatography , reaction 3h), the reaction mixture was cooled to room temperature, the reaction was quenched with 30mL of water, and 30mL of ethyl acetate was added for extraction, the organic phase was separated, and the aqueous phase was extracted with 30mL of ethyl acetate, the organic phases were combined and washed with anhydrous sulfuric acid Dry over magnesium, filter, and concentrate under reduced pre...
Embodiment 2
[0022] Preparation and characterization of 2-methyl-3-methoxy-1-(4-methyl)phenyl-1-propanone (2b):
[0023]
[0024] 4-Methylpropiophenone (0.5mmol, 1.0equiv), cesium carbonate (1.0mmol, 2equiv) and tetrabutylammonium tetrafluoroborate (0.25mmol, 0.5equiv) were placed in a 10mL three-neck round bottom flask, and the flask Equipped with a condenser, RVC (100ppi, 1cmx1cmx1.2cm) anode and platinum plate (1cmx1cm) cathode, put on an oxygen balloon and add 6mL methanol to the reaction bottle, and react at 65°C with a constant current of 10mA until the raw material is completely consumed (by TLC monitoring, reaction 3h), the reaction mixture was cooled to room temperature, quenched the reaction with 30mL water, and added 30mL ethyl acetate for extraction, separated the organic phase, and then extracted the aqueous phase with 30mL ethyl acetate, combined the organic phase After drying with anhydrous magnesium sulfate, filtering, and concentrating under reduced pressure, the residu...
Embodiment 3
[0028] Preparation and characterization of 2-methyl-3-methoxy-1-(4-methoxy)phenyl-1-propanone (2c):
[0029]
[0030] 4-Methoxypropiophenone (0.5mmol, 1.0equiv), potassium carbonate (1.0mmol, 2equiv) and tetrabutylammonium tetrafluoroborate (0.25mmol, 0.5equiv) were placed in a 10mL three-neck round bottom flask, The flask is equipped with a condenser, RVC (100ppi, 1cmx1cmx1.2cm) anode and platinum plate (1cmx1cm) cathode, put on an oxygen bulb and add 6mL of methanol to the reaction flask, and react at a constant current of 10mA at 65°C until the raw materials are completely consumed ( Monitored by thin-layer chromatography, reaction 4h), the reaction mixture was cooled to room temperature, quenched the reaction with 30mL of water, and added 30mL of ethyl acetate for extraction, separated the organic phase, and then extracted the aqueous phase with 30mL of ethyl acetate, combined organic The phases were combined and dried with anhydrous magnesium sulfate, filtered, and con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com