Supported imidazole ionic liquid catalyst and method for synthesizing 2-amino-3-cyano-4h-pyran compounds
A technology of imidazole ionic liquid and ionic liquid, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve by-product environmental pollution, low atom utilization, Problems such as high production cost, to achieve the effect of increasing product yield, promoting the positive movement of reaction balance, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
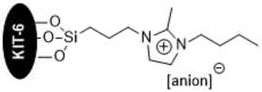
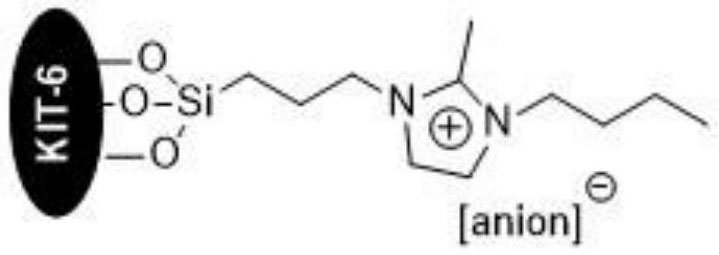
[0038] The preparation method of the KIT-6 loaded imidazolium ionic liquid of embodiment, comprises the following steps:
[0039] (1) N-methylimidazole, sodium ethoxide and ethanol solvent were reacted at 60-100°C for 7-10 hours, the solvent was recovered, and the product was dried to obtain intermediate 1;
[0040] (2) Intermediate 1, 3-chloropropyltriethoxysilane and acetonitrile solvent were reacted at 60-100°C for 10-20 hours, the solvent was recovered, and the product was dried to obtain Intermediate 2;
[0041] (3) Intermediate 2, n-bromobutane and toluene solvent were reacted at 80-120°C for 24-48 hours, the solvent was recovered, and the product was dried to obtain Intermediate 3;
[0042] (4) Intermediate 3, ionic liquid raw material and methanol solvent are reacted at 50-60° C. for 12-24 hours, the solvent is recovered, and the product is dried to obtain an ionic liquid;
[0043] (5) React ionic liquid 4, KIT-6 and ethanol solvent at 60-100° C. for 12-24 hours, reco...
Embodiment 1
[0047] In the reaction flask, add benzaldehyde (10mmol), malononitrile (10mmol), ethyl acetoacetate (10mmol), KIT-6 supported vanadate imidazole ionic liquid catalyst (0.5g), ethanol (20mL), oil bath Heating and stirring, the temperature is controlled at 80 ° C, and the stirring is continued for 3 hours after the temperature is stable. After the reaction is completed, the filtrate is recovered by filtration, the filter cake is washed, recrystallized, and dried to obtain 2-amino-3-cyano-4H-pyran. The yield is 51.4%.
Embodiment 2
[0049] In the reaction flask, add benzaldehyde (10mmol), malononitrile (10mmol), ethyl acetoacetate (10mmol), KIT-6 supported ethoxylate imidazole ionic liquid catalyst (0.5g), ethanol (20mL), oil bath Heat and stir, control the temperature at 50°C, continue stirring for 3 hours after the temperature is stable, filter and recover the filtrate after the reaction is completed, wash the filter cake, recrystallize and dry to obtain 2-amino-3-cyano-4H-pyran, the yield is 50.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com