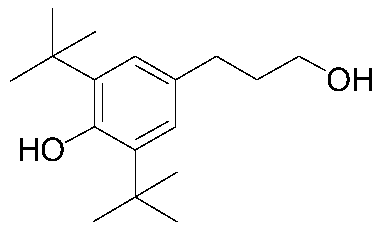
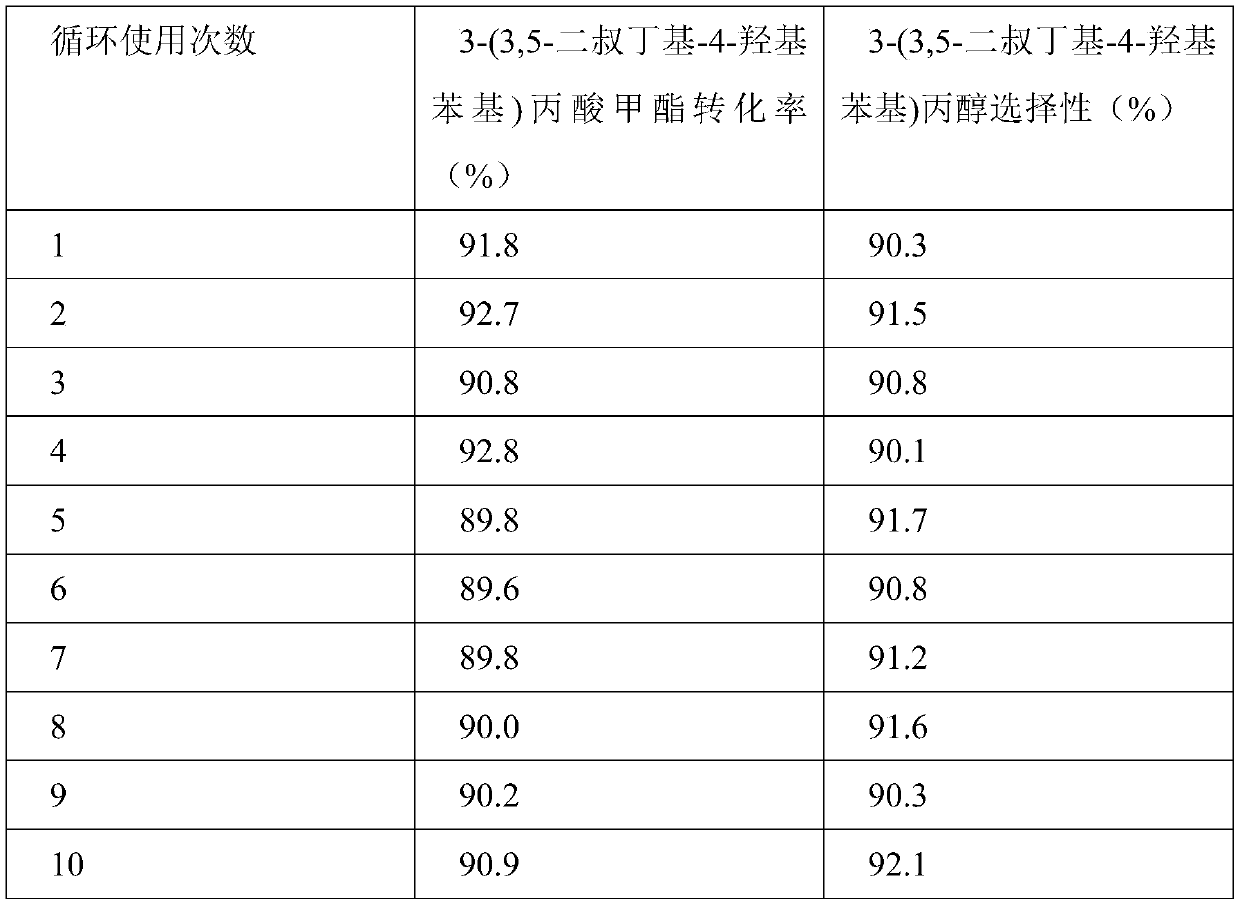
Catalyst for synthesizing 3-(3, 5-di-tert-butyl-4-hydroxyphenyl)propanol and preparation and application of catalyst
A technology of hydroxyphenyl and di-tert-butyl, which is applied in the field of catalysts for the synthesis of 3-propanol, can solve problems such as poor activity, carbon-carbon bond breakage of aromatic alcohols, and harsh reaction conditions, and achieve high conversion rate and high conversion rate and selectivity, the effect of excellent selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] Catalyst for the synthesis of 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanol by hydrogenation of methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate A preparation method comprising the steps of:
[0049] (1), take the soluble salt of the active component of formula quantity and the soluble salt of auxiliary agent precursor and dissolve in the solvent to obtain the salt solution;
[0050] (2), take the carrier of formula quantity and join in the saline solution of step (1), mix evenly, obtain mixed solution;
[0051] (3), drying the mixed solution of step (2) at room temperature for 5-120 hours;
[0052] (4), drying the product obtained in step (3) at 80-150°C for 2-60 hours;
[0053] (5), the product obtained in step (4) is roasted for 2 to 38 hours at 200 to 1000° C.;
[0054] (6), add the product obtained in step (5) into a solution containing cetyltrimethylammonium bromide, ammonia, 3-aminophenol, and formaldehyde, and stir at room temperature for 10 to 36 hours...
Embodiment 1
[0059] At room temperature, 3.8 g of copper nitrate and 0.2 g of palladium nitrate were dissolved in 10 mL of deionized water, and 10 g of silica carrier was weighed and placed in the above salt solution. Dry at room temperature for 24 hours, transfer to a blast dryer and dry at 100°C for 4 hours. Then the obtained product was baked at 500°C for 10 h in a muffle furnace. Get 0.5g of the resulting product and place it in a mixed solution containing 0.2g cetyltrimethylammonium bromide, 0.5ml ammonia water (25wt%), 0.1g 3-aminophenol, and 0.11ml formaldehyde, stir at room temperature for 18h, then heat up Stir at 100°C for 5h. After centrifugation, a solid product was obtained and dried at 120°C for 3 hours. The obtained catalyst is Cu-Pd / SiO 2 .
[0060] Take 0.5g of the above catalyst and place it in a high-temperature reduction furnace to activate the catalyst. The activation conditions are as follows: temperature is 300°C, pressure is 0.1MPa, reducing gas is hydrogen, and...
Embodiment 2
[0062] At room temperature, 3.8 g of copper nitrate and 0.2 g of chloroplatinic acid were dissolved in 10 mL of deionized water, and 10 g of silica carrier was weighed and placed in the above salt solution. Dry at room temperature for 24 hours, transfer to a blast dryer and dry at 100°C for 4 hours. Then the obtained product was baked at 500°C for 10 h in a muffle furnace. Get 0.5g of the resulting product and place it in a mixed solution containing 0.2g cetyltrimethylammonium bromide, 0.5ml ammonia water (25wt%), 0.1g 3-aminophenol, and 0.11ml formaldehyde, stir at room temperature for 18h, then heat up Stir at 100°C for 5h. After centrifugation, a solid product was obtained and dried at 120°C for 3 hours. The obtained catalyst is Cu-Pt / SiO 2 .
[0063] Take 0.5g of the above catalyst and place it in a high-temperature reduction furnace to activate the catalyst. The activation conditions are as follows: temperature is 300°C, pressure is 0.1MPa, reducing gas is hydrogen, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com