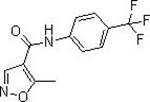
A kind of preparation method of leflunomide
A technology of leflunomide and trifluoromethylaniline, which is applied in the field of new preparation technology of pharmaceutical raw material leflunomide, can solve the problems of equipment corrosion, waste of raw materials, a large amount of industrial waste gas and acid waste water, etc., and achieves waste generation. Less liquid and less process pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 5-Methylisoxazole-4-carboxylic acid
[0034] Dissolve 50 g of ethyl acetoacetate in DMF-DMA, cool to -20°C, slowly add 10.1 g of sodium hydrogen, rise to room temperature and react for 3 hours, add water to precipitate a solid, stir, filter and dry to obtain a dimethylenamine structure. Ethyl N, N-dimethylaminomethylene acetoacetate was cooled to -5°C in ethanol, and 26.7 g of hydroxylamine hydrochloride was added in an equiproportional molar amount, and reacted at room temperature until the raw materials disappeared. After the reaction was completed, the solvent was spin-dried, and 30 % hydrochloric acid aqueous solution was heated to reflux, evaporated to dryness, and recrystallized from toluene to obtain 48 g of 5-methylisoxazole-4-carboxylic acid, with a yield of 98.3%.
[0035] Leflunomide
[0036] Put 20g of 5-methylisoxazole-4-carboxylic acid in dichloromethane, cool to 0°C, add triethylamine and CDI25.5g, stir for half an hour, then add 25.3g of 4-trifluorometh...
Embodiment 2
[0038] 5-Methylisoxazole-4-carboxylic acid
[0039] Dissolve 50 g of ethyl acetoacetate in DMF-DMA, cool to -20°C, slowly add 10.1 g of sodium hydrogen, rise to room temperature and react for 3 hours, add water to precipitate a solid, stir, filter and dry to obtain a dimethylenamine structure. Ethyl N, N-dimethylaminomethylene acetoacetate was cooled to -5°C in ethanol, and 26.7 g of hydroxylamine hydrochloride was added in an equiproportional molar amount, and reacted at room temperature until the raw materials disappeared. After the reaction was completed, the solvent was spin-dried, and 30 % hydrochloric acid aqueous solution was heated to reflux, evaporated to dryness, and recrystallized from toluene to obtain 48 g of 5-methylisoxazole-4-carboxylic acid, with a yield of 98.3%.
[0040] Leflunomide
[0041]Put 20g of 5-methylisoxazole-4-carboxylic acid in dichloromethane, cool to 0°C, add triethylamine and CDI38.3g, stir for half an hour, add 25.3g of 4-trifluoromethylanil...
Embodiment 3
[0043] 5-Methylisoxazole-4-carboxylic acid
[0044] Dissolve 50 g of ethyl acetoacetate in DMF-DMA, cool to -20°C, slowly add 10.1 g of sodium hydrogen, rise to room temperature and react for 3 hours, add water to precipitate a solid, stir, filter and dry to obtain a dimethylenamine structure. Ethyl N, N-dimethylaminomethylene acetoacetate was cooled to -5°C in ethanol, and 26.7 g of hydroxylamine hydrochloride was added in an equiproportional molar amount, and reacted at room temperature until the raw materials disappeared. After the reaction was completed, the solvent was spin-dried, and 30 % hydrochloric acid aqueous solution was heated to reflux, evaporated to dryness, and recrystallized from toluene to obtain 48 g of 5-methylisoxazole-4-carboxylic acid, with a yield of 98.3%.
[0045] Leflunomide
[0046] Put 20g of 5-methylisoxazole-4-carboxylic acid in dichloromethane, cool to 0°C, add triethylamine and CDI25.5g, stir for half an hour, add 38g of 4-trifluoromethylanili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com