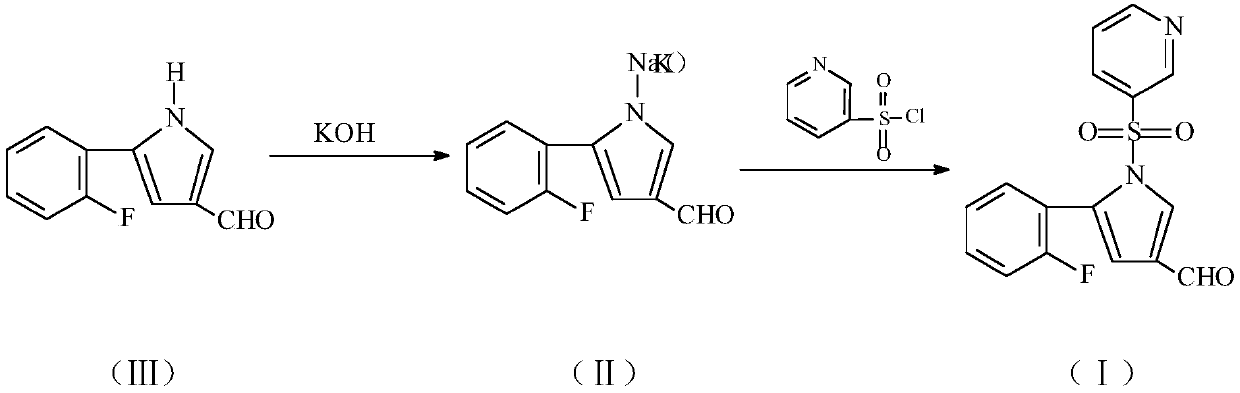
Preparation and purification method of 5-(2-fluorophenyl)-1-(pyridine-3-ylsulfonyl)-1H-pyrrole-3-formaldehyde
A technology for the preparation of sulfonyl and formaldehyde, which is applied in the field of chemical synthesis and preparation, can solve the problems of complex processing, high production cost, and many reaction by-products, and achieve the effects of easy control of process conditions, low production cost, and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A. In a 10L glass-lined reactor equipped with a stirrer, a thermometer, a vacuum pressure gauge, etc., add 4725g of acetonitrile and 189g of compound (Ⅲ) under stirring, cool down to 15°C, add 140g of potassium hydroxide powder, and replace the air with nitrogen Afterwards, heat preservation and stirring reaction for 1.5 hours. Then, 397.7 g of pyridine-3-sulfonyl chloride was added dropwise to the reaction liquid, the reaction temperature was controlled at 30±2° C., and the reaction was carried out while insulated and stirred for 1 hour. Hydraulically filter the reaction mixture into the still, and recover the solvent by distillation under reduced pressure until there is no distillate. The internal temperature is controlled below 50°C, and the vacuum degree is ≥-0.07MPa. After the concentration is completed, add 472.5g ethyl acetate and 2362.5g n-hexane Add 13 g of activated carbon under stirring, heat up to 70°C for decolorization for 0.5 hours, press filter, cool dow...
Embodiment 2
[0021] A. In a 10L glass-lined reactor equipped with a stirrer, a thermometer, a vacuum pressure gauge, etc., add 4725g of acetonitrile and 189g of compound (Ⅲ) under stirring, cool down to 10°C, add 168g of potassium hydroxide powder, and replace the air with nitrogen , keep stirring and react for 2 hours. Then add 477.2g of pyridine-3-sulfonyl chloride dropwise to the reaction solution, control the reaction temperature at 30±2°C, keep the temperature and stir for 0.5 hours, filter the reaction mixture into the still, and recover the solvent by distillation under reduced pressure until there is no distillate Until the internal temperature is controlled below 50°C, the vacuum degree is ≥-0.07MPa, after the concentration is completed, add 405g of ethyl acetate and 2430g of n-hexane, add 11.3g of activated carbon under stirring, heat up to 70°C for decolorization for 0.5 hours, press filter, cool down and crystallize Heat it at 3°C for 1.5 hours to crystallize, centrifuge, and...
Embodiment 3
[0024] A. In a 10L glass-lined reactor equipped with a stirrer, a thermometer, a vacuum pressure gauge, etc., add 2835g of acetonitrile and 189g of compound (Ⅲ) under stirring, cool down to 12°C, add 84g of potassium hydroxide powder, and replace the air with nitrogen , keep stirring and react for 2 hours. Add 238.6g of pyridine-3-sulfonyl chloride dropwise to the reaction, control the reaction temperature at 30±2°C, keep the temperature and stir for 1.5 hours, filter the reaction mixture into the still, and recover the solvent by distillation under reduced pressure until there is no distillate , the internal temperature is controlled below 50°C, the vacuum degree is ≥-0.07MPa, after the concentration is completed, add 472.5g of ethyl acetate and 1417.5g of n-hexane, add 9.5g of activated carbon under stirring, heat up to 75°C for 0.5 hours of decolorization, press filter, and cool down The crystallization was carried out at 0° C., incubated for 1 hour, centrifuged, and vacuum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com