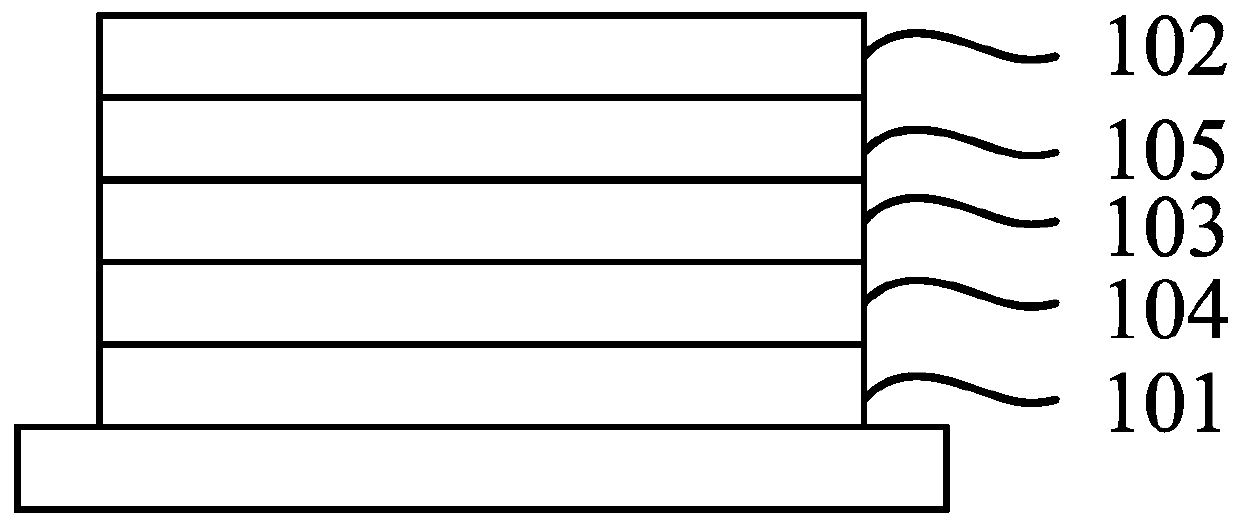
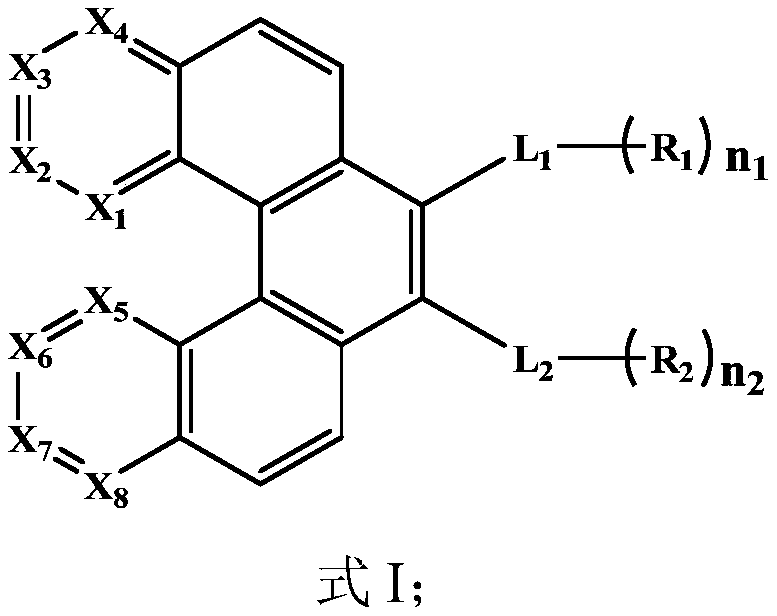
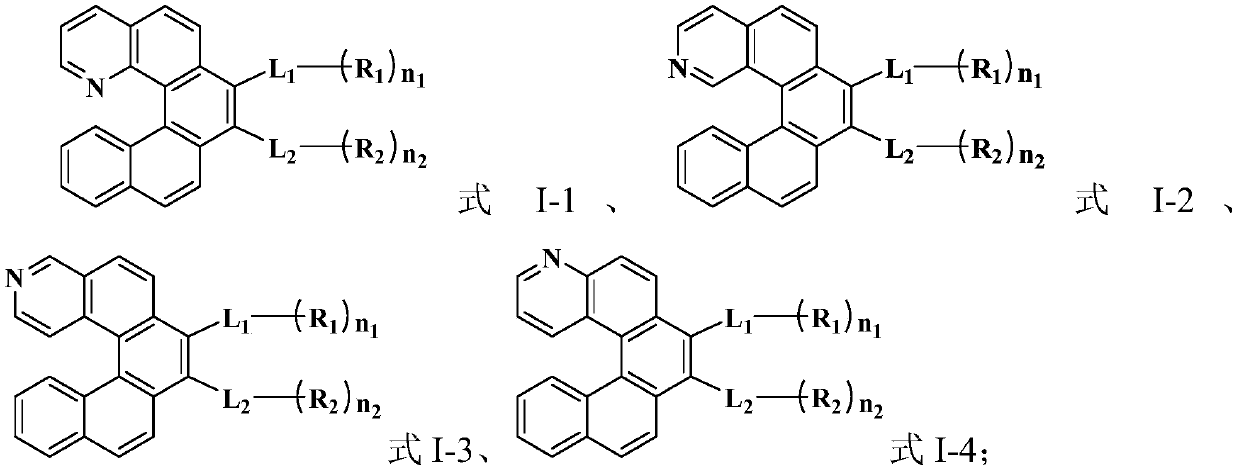
Organic compound, organic electroluminescent material and application thereof
An organic compound and selected technology, applied in the field of organic electroluminescent materials and organic compounds, can solve the problems of reduced electron transport performance, reduced exciton formation efficiency, low glass transition temperature, etc. The effect of hole blocking ability, improving luminous efficiency and performance stability, and excellent film stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] This embodiment provides an organic compound with the following structure:
[0103]
[0104] The preparation method of this organic compound M1 comprises the following steps:
[0105]
[0106] Under a nitrogen atmosphere, add 100mL of 1,4-dioxane solvent into a 250mL reaction flask, and then add K 2 CO 3 (9mmol), 7-methyl-8-bromoquinoline (3mmol), 7-methyl-8-boronic acid quinoline (3mmol), and palladium catalyst Pd (PPh 3 ) 4 (0.15 mmol), heated to 100°C, and reacted overnight. After the reaction is completed, cool to room temperature, add dichloromethane DCM and H 2 O was extracted, and the collected organic phase was washed with anhydrous Na 2 SO 4 Dry, collect the filtrate by suction filtration, spin off the solvent and perform column chromatography purification to obtain intermediate 1-1;
[0107] Test the structure of intermediate 1-1: m / z obtained by liquid chromatography-mass spectrometry (LC-MS): C 20 h 16 N 2 , the calculated value is 284.35, an...
Embodiment 2
[0119] This embodiment provides an organic compound with the following structure:
[0120]
[0121] The preparation method of this organic compound M301 comprises the following steps:
[0122] (1) The synthetic method of intermediate 1-3 is the same as in Example 1;
[0123]
[0124] Under nitrogen atmosphere, intermediate 1-3 (1 mmol) and zinc powder (5 mmol) were added to 30 mL of acetic acid, the reaction mixture was heated to 130° C., and refluxed for 5 h; the mixture solution gradually became transparent. After the reaction is finished, filter the mixture while it is hot to remove excess zinc; the filtrate is cooled, and as the acetic acid is cooled to room temperature, a solid is precipitated, the crude solid is filtered, and recrystallized with methanol, suction filtered and the filter cake is vacuum-dried to obtain Intermediate 2-1.
[0125] Tested the structure of Intermediate 2-1: LC-MS gave m / z: C 20 h 11 BrN 2 , the calculated value is 359.22, and the me...
Embodiment 3
[0131] This embodiment provides an organic compound with the following structure:
[0132]
[0133] The preparation method of this organic compound M302 comprises the following steps:
[0134] (1) The synthetic method of intermediate 2-1 is the same as in Example 2;
[0135]
[0136] Under a nitrogen atmosphere, add 100mL of 1,4-dioxane solvent into a 250mL reaction flask, and then add K 2 CO 3 (2mmol), intermediate 2-1 (1mmol), 4'-boronic acid-[3,2'; 6',3"]tripyridine (1.2mmol) and Pd(PPh 3 ) 4 (0.05 mmol), heated to 100°C, and reacted overnight. After the reaction is completed, cool to room temperature, add DCM, H 2 O was extracted, and the collected organic phase was washed with anhydrous Na 2 SO 4 After drying, the filtrate was collected by suction filtration, the solvent was spun off and purified by column chromatography to obtain the target product M302.
[0137] Tested the structure of M302: LC-MS got m / z: C 35 h 21 N 5 , the calculated value is 511.57,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com