Preparation method of naphthotriazolodiazepineketone compound
A technology of naphthotriazole and oxazone, which is applied in the field of organic synthesis, can solve the problems of being unsuitable for large-scale production, harsh reaction conditions, and a limited range of substrates, and achieves high yield, simple operation, and short reaction time. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
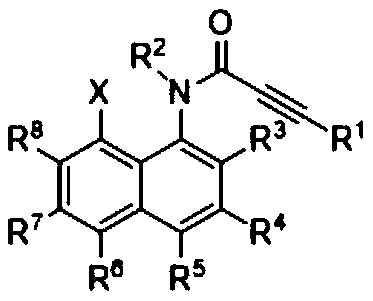
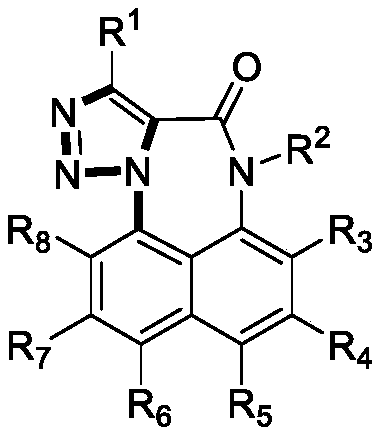
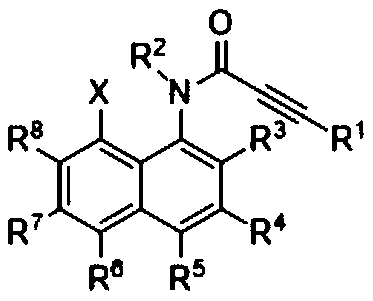
[0024] Specific embodiment one: the method for synthesizing naphthotriazolodiazepinone by N-(8-X-naphthalene-1-yl) propyne amide (2) in this embodiment is carried out according to the following steps:
[0025] Taking N-(8-X-naphthalene-1-yl) propyne amide (2) as the starting material (wherein, X=Br, I), reacted with sodium azide under heating conditions in an appropriate solvent, and was thinned by TLC The laminate monitors whether the reaction is complete, and the target compound naphthotriazolodiazepine (1) can be obtained after separation and purification.
[0026] Wherein said N-(8-X-naphthalene-1-yl) propynamide (2) is substituent R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 for C 1 ~C 16 Alkyl, alkenyl, alkynyl, aryl, halogen, cyano, trifluoromethyl, trifluoromethylsulfonyl, and five- and six-membered heterocycles containing oxygen, sulfur, and nitrogen.
specific Embodiment approach 2
[0027] Specific embodiment 2: This embodiment differs from specific embodiment 1 or 2 in that the molar ratio of N-(8-X-naphthalen-1-yl) propynamide (2) to sodium azide is (1-2): (1~6).
specific Embodiment approach 3
[0028] Specific embodiment three: This embodiment differs from specific embodiments one to four in that the reaction solvent is dioxane, dimethyl sulfoxide (DMSO), N,N-dimethylformamide (DMF), N- Methylpyrrolidone (NMP), N,N-Dimethylacetamide (DMAc), N,N-Dimethylpropenylurea (DMPU).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com