Recombinant yarrowia lipolytica for heterogeneous synthesis of alpha-amyrin and ursolic acid and construction method
A technology of Yarrowia lipolytica and balsamic resin is applied in the biological field to achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1. Construction of Heterologous Synthesis of α-Amyrin Recombinant Yarrowia lipolytica YL-AAS (Recombinant Bacteria 1)
[0046] 1. Module construction
[0047] The mixedAS gene (GenBank: AFJ19235.1) from the plant Vinca (Catharanthus roseus) was codon optimized and fully chemically synthesized by Wuhan Jinkairui Company for Yarrowia lipolytica, and the optimized mixed arysin alcohol synthesis Enzyme encoding gene, the nucleotide sequence of the optimized mixed balsam alcohol synthase encoding gene is shown in SEQ ID NO.1;
[0048] Promoter TEF1 (SEQ ID NO.14), terminator XPR2 (SEQ ID NO.17), rDNA-up (SEQ ID NO.22), rDNA-down (SEQ ID NO.23) are all from Yarrowia lipolytica ATCC 201249 genome, the screening marker gene URA3 (SEQ ID NO.20) sequence is from Yarrowia lipolytica ATCC 201249, which was synthesized by Wuhan Jinkairui Bioengineering Co., Ltd. through chemical synthesis;
[0049] Using Yarrowia lipolytica ATCC201249 genome as a template,
[0050] Use rD...
Embodiment 2
[0056] Example 2, Construction of recombinant Yarrowia lipolytica YL-UA (recombinant bacteria 2) for heterologous synthesis of α-amyresinol and ursolic acid
[0057] The mixedAS gene (GenBank: AFJ19235.1) from the plant Vinca (Catharanthus roseus) was codon optimized and fully chemically synthesized by Wuhan Jinkairui Company for Yarrowia lipolytica, and the optimized mixed arysin alcohol synthesis Enzyme encoding gene, the nucleotide sequence of the optimized mixed balsam alcohol synthase encoding gene is shown in SEQ ID NO.1;
[0058] CYP716A12 (GenBank: CBN88268.1) derived from Medicago truncatula (GenBank: CBN88268.1) was codon optimized and fully chemically synthesized by Wuhan Jinkairui Company for Yarrowia lipolytica, and the optimized ursolic acid synthase encoding gene was obtained. The optimized nucleotide sequence of the gene encoding ursolic acid synthase is shown in SEQ ID NO.2.
[0059] The gene encoding cytochrome-NADPH-reductase 1, AtCPR1, was derived from Ara...
Embodiment 3
[0068] Example 3, gene element cloning and construction of plasmids containing corresponding gene elements
[0069] 1. PCR amplification of genetic elements
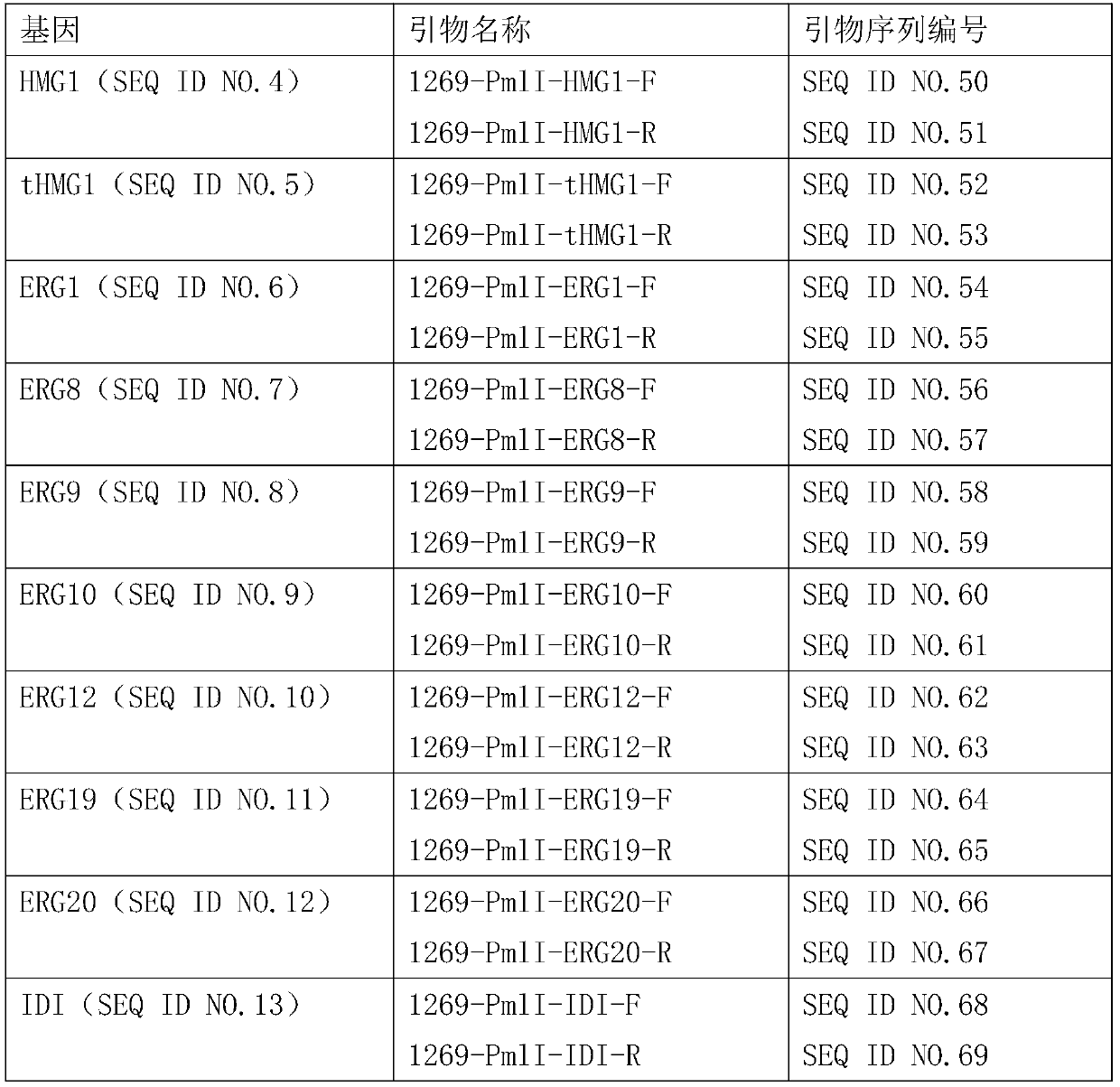
[0070] Using the Yarrowia lipolytica ATCC201249 genome as a template, HMG1, tHMG1, ERG1, ERG8, ERG9, ERG10, ERG12, ERG19, ERG20 and IDI were amplified with the primers in Table 1, respectively. The gene fragments obtained after amplification were purified and recovered for future use.
[0071] Table 1 PCR amplification gene primer list
[0072]
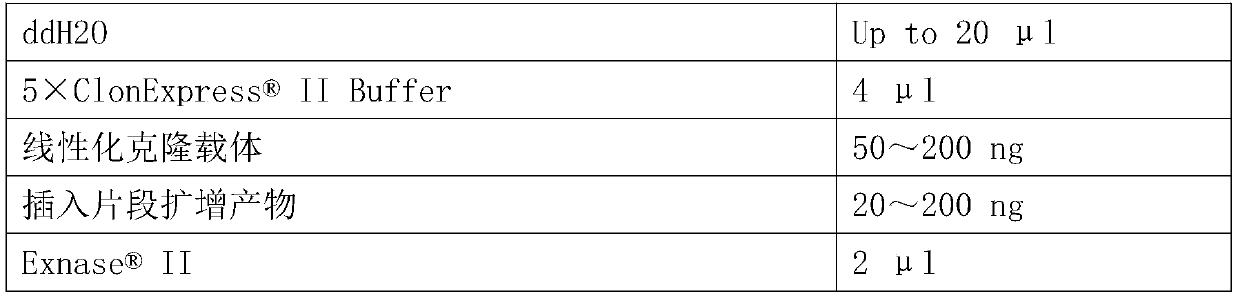
[0073] The PCR enzyme used in the present invention is from Nanjing Nuoweizan Biotechnology Co., Ltd. Max Super-Fidelity polymerase. The 50 μL PCR amplification system is as follows: DNA template, 1 μL; 2 μL each of the front primer (10 μM) and the back primer (10 μM); dNTP (10 mM), 1 μL; 2×Phanta Max Buffer, 25 μL; Max Super-Fidelity polymerase, 1 μL; finally make up 50 μL with double distilled water. Set up the amplification program on the PCR machine. Amplificati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com