Method for detecting chromium content of high-chromium-content aluminum alloy based on titration analysis method
An analytical method, an aluminum alloy technology, applied in the field of chemical analysis, which can solve the problems of high price, limited use conditions, and inability to guarantee the accuracy of the results, and achieves the effects of simple operation, few interfering elements, and fast chemical reaction speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
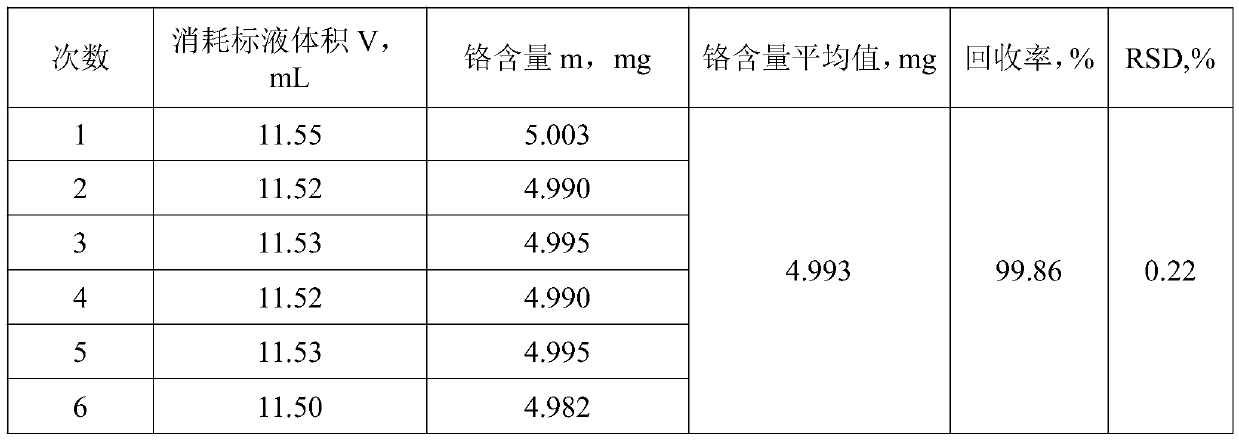
[0060] Weigh 0.5000g of an aluminum alloy sample that does not contain manganese and place it in a reactor, add 20mL of sodium hydroxide solution into the reactor, and when the action is slow, add 10 drops of hydrogen peroxide dropwise into the reactor until the sample is completely dissolved Finally, heat on an electric furnace at 250°C to decompose hydrogen peroxide, remove the heat source, add 25mL of nitric acid to acidify the reactor after it cools down slightly, then add 20mL of sulfuric acid-phosphoric acid mixed medium, and then add 200mL of water for dilution. When the chromium ion is in a stable state, add 8 mL of oxidant to the mixed solution, add 5 drops of silver nitrate solution, and then add 5 drops of manganese sulfate solution, until the color of the mixed solution turns purple, heat and boil until the oxidant is completely decomposed, After cooling, add 5mL hydrochloric acid solution, continue heating and boiling for 7min after boiling, and cool the solution t...
Embodiment 2
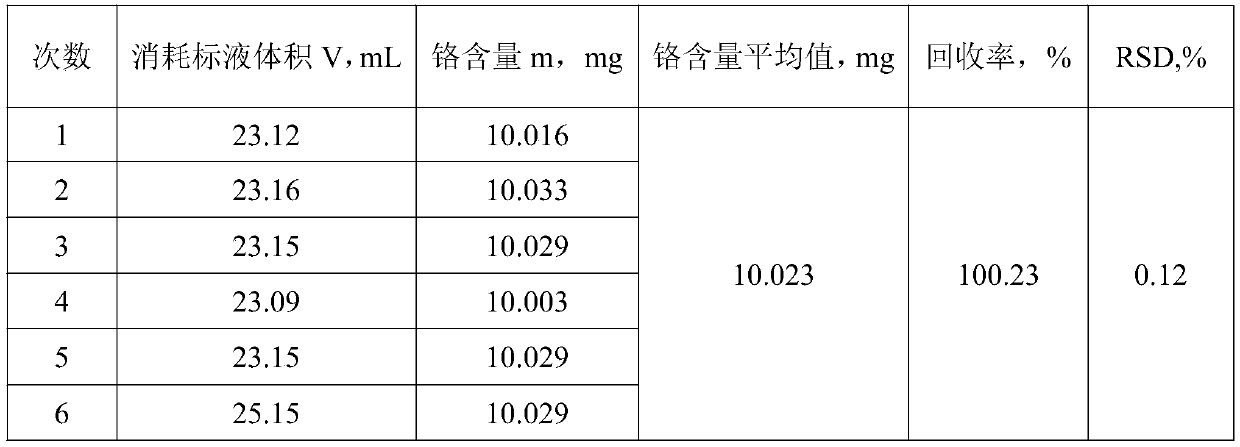
[0073] Weigh 0.5000g of an aluminum alloy sample that does not contain manganese and place it in a reactor, add 20mL of sodium hydroxide solution into the reactor, and when the action is slow, add 10 drops of hydrogen peroxide dropwise into the reactor until the sample is completely dissolved Finally, heat on an electric furnace at 250°C to decompose hydrogen peroxide, remove the heat source, add 25mL of nitric acid to acidify the reactor after it cools down slightly, then add 20mL of sulfuric acid-phosphoric acid mixed medium, and then add 200mL of water to dilute. The trivalent chromium ion is in a stable state. Add 8 mL of oxidant to the mixed solution, add 5 drops of silver nitrate solution, and then add 5 drops of manganese sulfate solution. When the color of the mixed solution turns purple, heat and boil until the oxidant is completely decomposed. After cooling down slightly, add 5mL of hydrochloric acid solution, continue heating and boiling for 8 minutes after boiling, ...
Embodiment 3
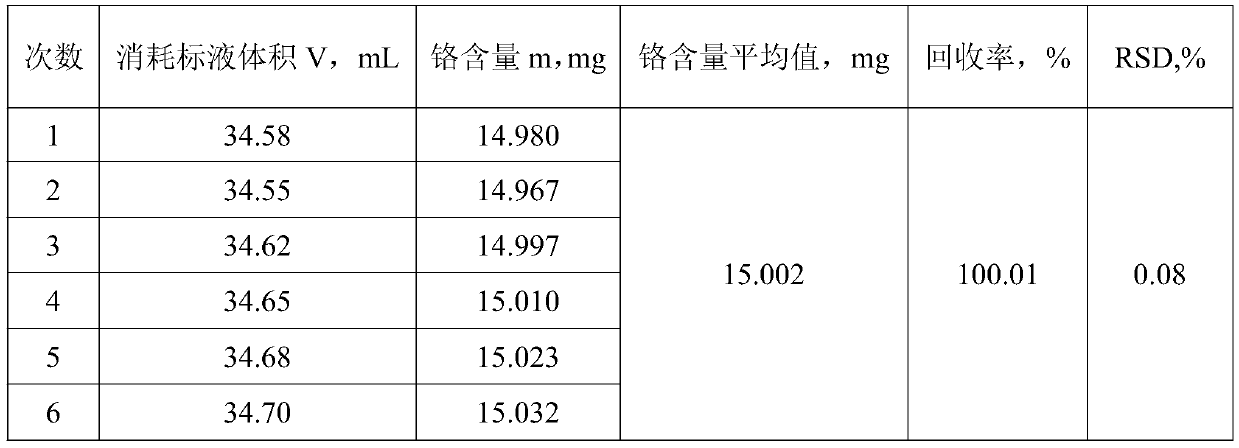
[0080] Weigh 0.5000g of an aluminum alloy sample containing manganese and place it in a reactor, add 20mL of sodium hydroxide solution into the reactor, and when the action is slow, add 10 drops of hydrogen peroxide dropwise into the reactor, and wait until the sample is completely dissolved , heated on an electric furnace at 250°C to decompose hydrogen peroxide, remove the heat source, add 25mL of nitric acid to acidify the reactor after it cools down slightly, then add 20mL of sulfuric acid-phosphoric acid mixed medium, and then add 200mL of water to dilute, at this time the trivalent When the chromium ion is in a stable state, add 8 mL of oxidant to the mixed solution, then dropwise add 5 drops of silver nitrate solution, heat and boil until the oxidant is completely decomposed, after cooling slightly, add 5 mL of hydrochloric acid solution, continue to boil for 7 minutes after boiling, and cool the solution to room temperature;
[0081] 2) Titration
[0082] Titrate with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com