Synthesis method for preparing saccharin
A synthesis method and saccharin technology, applied in organic chemistry and other directions, can solve the problems of many production links, long production process routes, and high ammonia nitrogen emissions, and achieve the effects of reducing emissions and treatment costs, reducing production costs, and avoiding process operations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
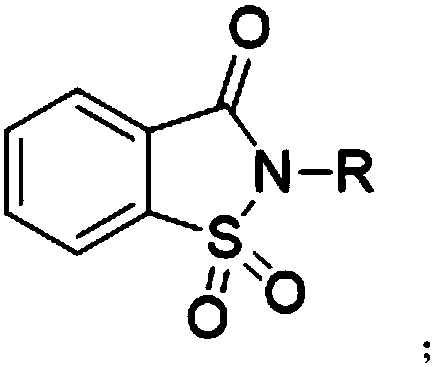
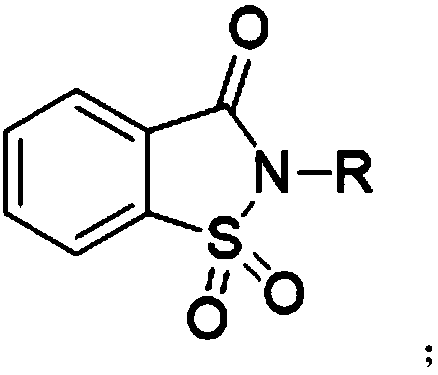
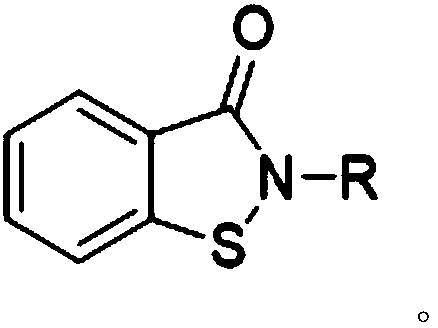
[0025] The synthetic method of preparing saccharin proposed by the present invention, its core is to carry out oxidation reaction of 1,2-benzisothiazolin-3-one compound and oxidizing agent, and oxidizing agent oxidizes 1,2-benzisothiazolin-3-one The thioether of compound is oxidized into sulfuramide, obtains o-benzoylsulfamide compound, and its reaction formula:
[0026]
[0027] Wherein, the R substituent is H or C 1 -C 8 Any substituent in straight-chain alkyl or branched-chain alkyl.
[0028] When specifically implementing the operation, the preferred embodiment can be carried out according to the following steps:
[0029] S1: Dissolve 1,2-benzisothiazolin-3-one compounds, salts, and alkalis in an organic solvent, stir, slowly add the oxidant dropwise, and stir after the dropwise addition is completed;
[0030] S2: Evaporate the solvent, add water, add acid dropwise, and adjust the pH value to 3-5;
[0031] S3: extracting the solution obtained in step S2, combining t...
Embodiment 1
[0034] Dissolve 15.1g (0.10mol) 1,2-benzisothiazolin-3-one, 0.206g (0.001mol) ruthenium trichloride, 4.0g (0.1mol) sodium hydroxide in 300mL ethanol, stir, drop slowly Add peracetic acid (20 mL). After 30 minutes of adding dropwise, stir for 6 hours. Evaporate the solvent, add 100mL of water, add hydrochloric acid dropwise, and adjust the pH value to 3. Extract 200mL×3 with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to obtain brown-yellow o-benzoylsulfonimide (C 7 h 5 NO 3 Crude product of S), 19.7 g. The crude product was recrystallized with 150 mL of ethanol to obtain 10.6 g of light yellow solid, yield: 57.9%.
Embodiment 2
[0036] Dissolve 15.1g (0.10mol) of 1,2-benzisothiazolin-3-one, 0.230g (0.001mol) of silver oxide, and 4.0g (0.1mol) of potassium hydroxide in 300mL of acetonitrile, stir, and slowly drop over Hydrogen peroxide (20 mL). After 30 minutes of adding dropwise, stir for 6 hours. The solvent was evaporated, 100mL of water was added, and hydrochloric acid was added dropwise to adjust the pH value to 5. Extract 200mL×3 with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, filter with suction, and concentrate the filtrate to obtain brown-yellow o-benzoylsulfonimide (C 7 h 5 NO 3 The crude product of S) 19.2 g. The crude product was recrystallized with 150 mL of ethanol to obtain 9.6 g of light yellow solid, yield: 52.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com