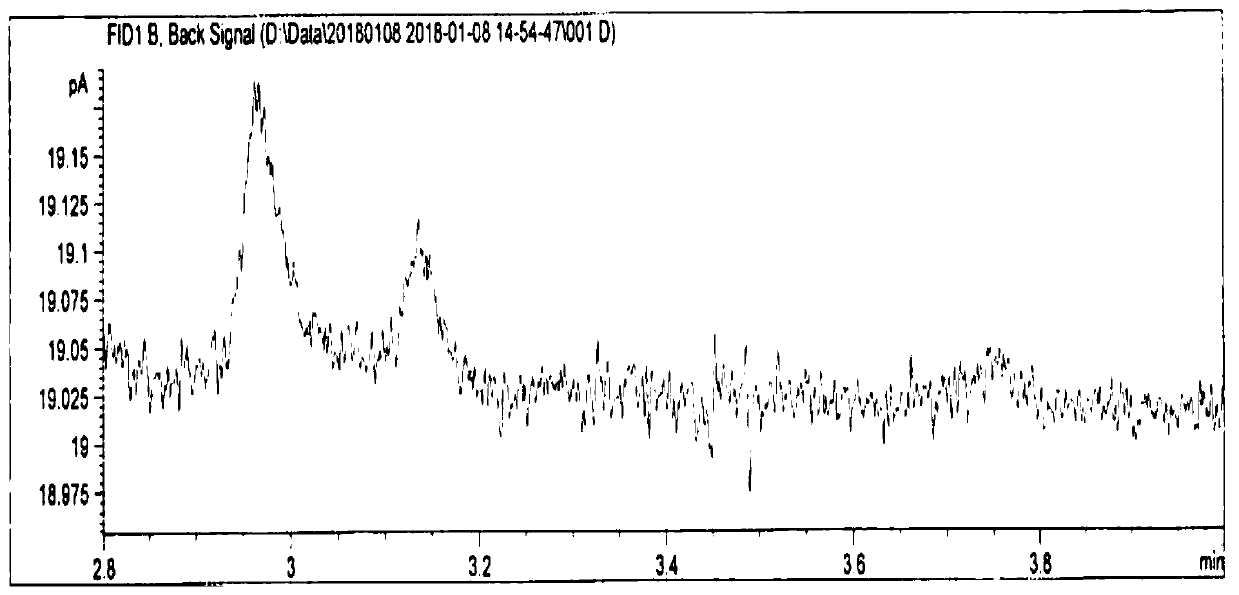
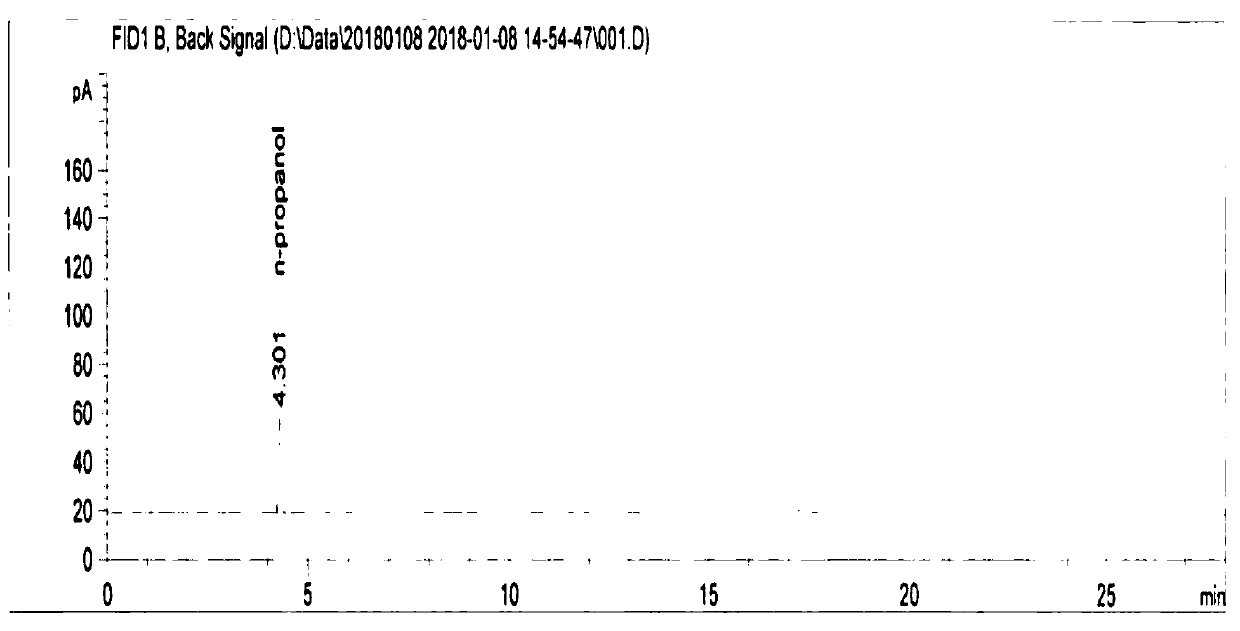
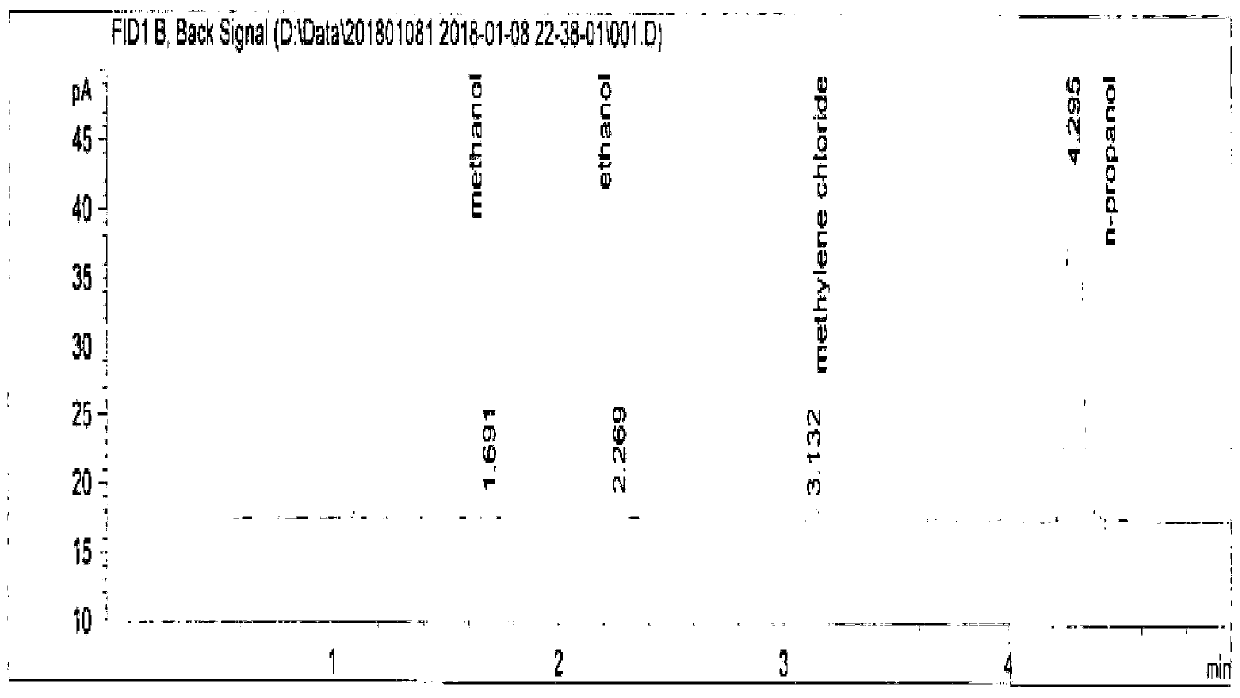
Method for analyzing residual solvent in enoxaparin sodium
A technology for enoxaparin sodium and residual solvents, which is applied in the field of analytical chemistry, can solve errors, cannot accurately analyze the residual levels of methanol, ethanol and dichloromethane in enoxaparin sodium, and qualitatively identify residual organic solvents in enoxaparin sodium and long quantitative analysis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Limits of detection and quantitation:
[0046] 1. Solution Preparation
[0047] 50% dimethyl sulfoxide stock solution: take 300 ml of water and 300 ml of dimethyl sulfoxide, put them in a 1000 ml beaker, stir well, and cool to room temperature.
[0048] Internal standard solution: take 50 μl of n-propanol, put it in a 500ml volumetric flask containing about 200ml of 50% dimethyl sulfoxide solution, add 50% dimethyl sulfoxide solution to dilute to the mark, and shake well to obtain 80 μg / ml of n-propanol solution.
[0049] Blank solution: Pipette 5.0ml of internal standard solution precisely, place it in a 20ml headspace bottle, and seal it.
[0050] Methanol control stock solution: Take about 0.3g of methanol, weigh it accurately, put it into a 50ml volumetric flask containing about 20ml of internal standard solution, dilute the internal standard solution to the mark, and shake well.
[0051] Ethanol control stock solution: Take about 0.5g of ethanol, weigh it accura...
Embodiment 2
[0071] Accuracy experiment:
[0072] 1. Solution Preparation
[0073] 50% dimethyl sulfoxide solution: take 600 ml of water and 600 ml of dimethyl sulfoxide, put them in a 2000 ml beaker, stir evenly, and cool to room temperature.
[0074] Internal standard solution: take 100 μl of n-propanol, put it in a 1000ml volumetric flask of about 500ml 50% dimethyl sulfoxide solution, add 50% dimethyl sulfoxide solution to dilute to the mark, shake well to get 80 μg / ml n-propanol solution.
[0075] Blank solution: Pipette 5.0ml of internal standard solution precisely, place it in a 20ml headspace bottle, and seal it.
[0076] Stock solution 1: Take about 0.3g of methanol, weigh it accurately, put it into a 50ml volumetric flask containing about 20ml of internal standard solution, add internal standard solution to dilute to the mark, and shake well.
[0077] Stock solution 2: Take about 0.5g of ethanol, weigh it accurately, put it into a 50ml volumetric flask containing about 20ml of...
Embodiment 3
[0089] Durability test:
[0090] When evaluating small changes in the parameters of the determination conditions by changing the headspace temperature, different flow rates, different column temperatures, and different chromatographic columns, the system applicability should meet the following requirements:
[0091] The degree of separation between each peak in the control solution should be greater than 1.5; the number of theoretical plates of methanol, ethanol, dichloromethane, and n-propanol peaks in the control solution should not be less than 5000; Chloromethane and the relative standard deviation of the internal standard peak area ratio are all no more than 5.0%; The relative standard deviations of the peak area ratios of n-propanol were not more than 5.0%.
[0092]
[0093] 1. Solution Preparation
[0094] 50% dimethyl sulfoxide solution: take 300 ml each of water and dimethyl sulfoxide, put them in a 1000 ml beaker, stir evenly, and cool to room temperature.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com