Stereoselective synthesis method of (Z, E)-13, 15-octadecenal
An octadecadienal and stereoselective technology, which can be used in organic chemistry methods, carbon-based compound preparation, chemical instruments and methods, etc., can solve problems such as inability to meet application requirements, and achieves low cost, simple purification, and raw materials. easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
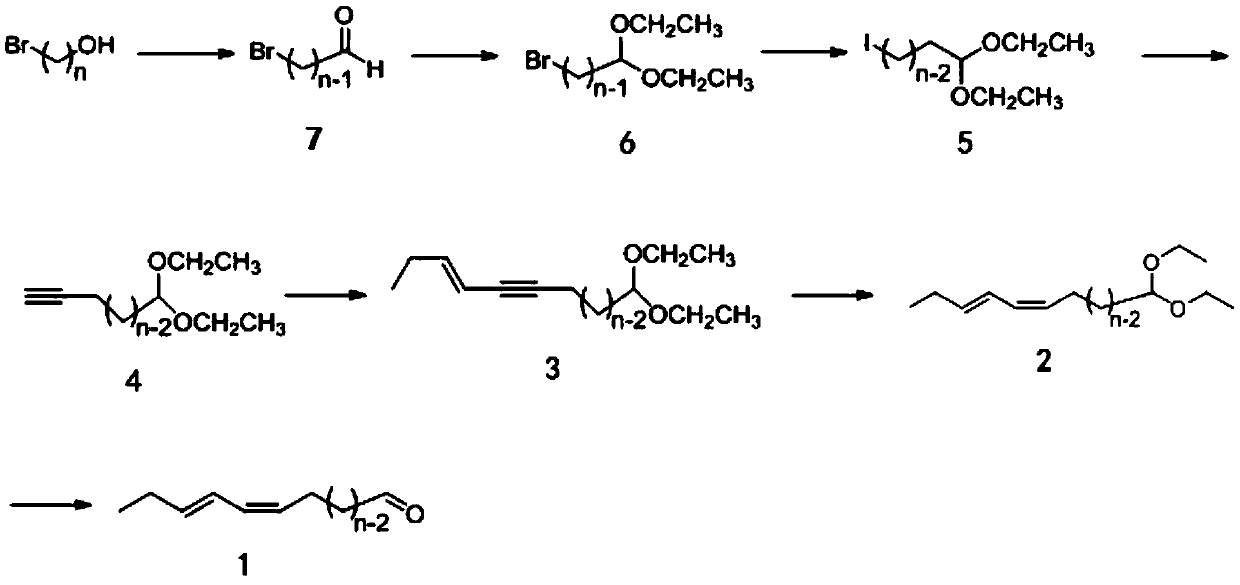
[0032] The invention provides a stereoselective synthesis method of (Z,E)-13,15-octadecadienal, comprising the following steps:
[0033] (1) Under the action of an oxidizing agent, 12-bromo-1-dodecanol is oxidized to obtain 12-bromo-1-dodecanal;
[0034] (2) Under the action of a catalyst, 12-bromo-1-dodecanal and triethyl orthoformate are subjected to an aldehyde protection reaction to obtain 1,1-diethoxy-12-bromododecane;
[0035] (3) Iodination reaction of sodium iodide and 1,1-diethoxy-12-bromododecane to obtain 1,1-diethoxy-12-iodododecane;
[0036] (4) Under the action of n-butyllithium, trimethylsilylacetylene and 1,1-diethoxy-12-iodododecane are subjected to a coupling reaction, and then removed under the action of potassium carbonate and methanol aqueous solution Coupling of the trimethylsilyl group in the reaction product yields 14,14-diethoxy-tetradec-1-yne;
[0037] (5) Under the catalysis of tetrakis(triphenylphosphine)palladium and cuprous iodide, the Sonogashi...
Embodiment 1
[0088] (1) Synthesis of 1,1-diethoxy-12-iodododecane
[0089] Add 12-bromo-1-dodecanol (26.5g, 0.1mol) dropwise into a solution of pyridinium chlorochromate (0.4mol) in dichloromethane (600mL) and react at room temperature for 4h to generate 12-bromo-1- Dodecanal;
[0090] The product was reacted with triethyl orthoformate (22.2g, 0.15mol) in absolute ethanol without purification, the catalyst was p-toluenesulfonic acid monohydrate (290mg), and reacted overnight at 0°C. After the reaction was completed, in the reaction solution Add saturated sodium bicarbonate solution, extract three times with ethyl acetate, combine the extracts, dry over anhydrous sodium sulfate, and evaporate the solvent to obtain the product 1,1-diethoxy-12-bromodeca dioxane;
[0091] In anhydrous acetone, 1,1-diethoxy-12-bromododecane was reacted with sodium iodide at a molar ratio of 2:1, and the bromine group was replaced by iodine; after the reaction, the organic solvent was distilled off , added di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com