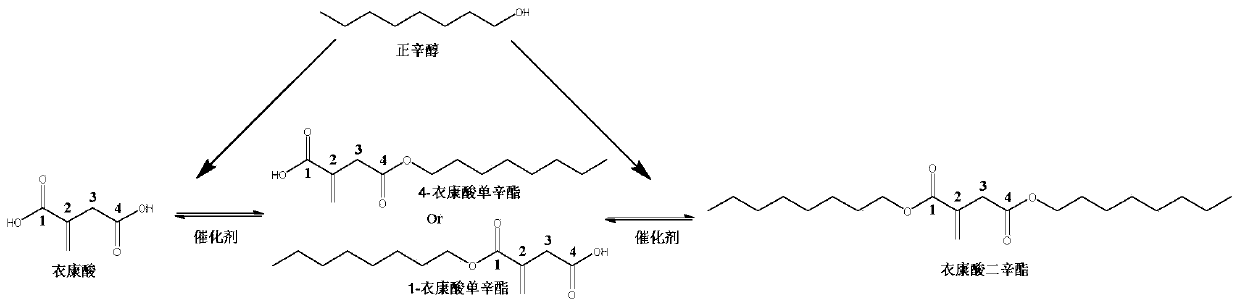
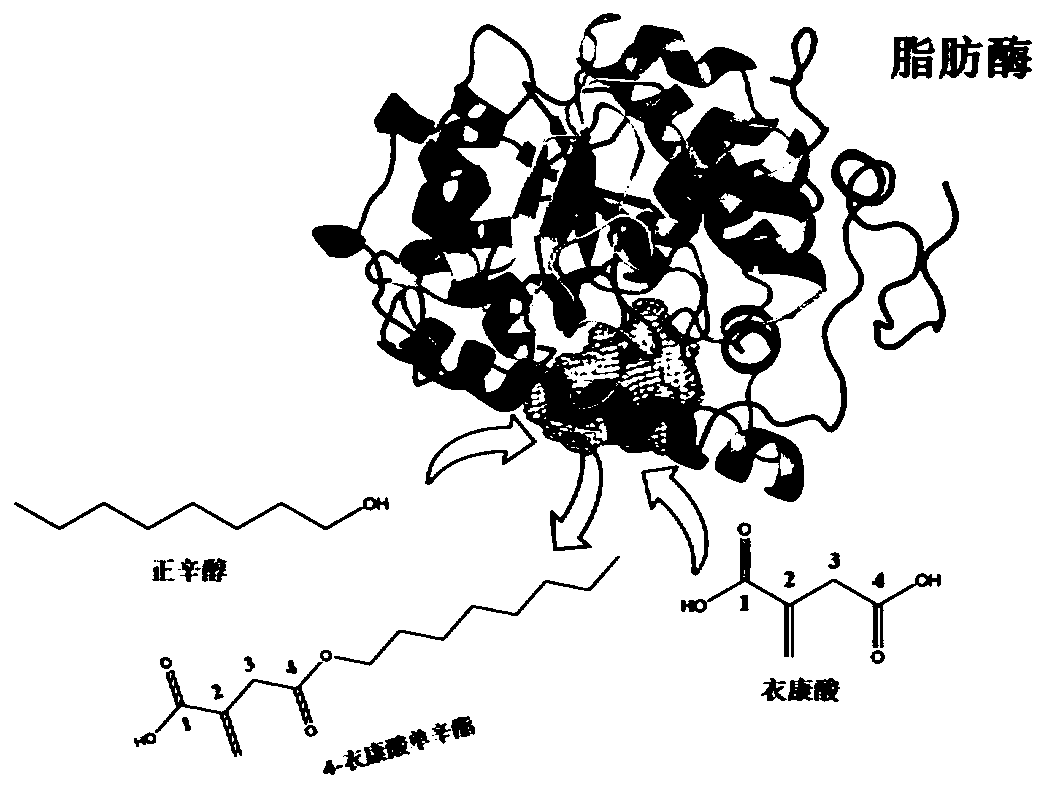
Method for preparing 4-octyl itaconate by enzymatic selective catalysis
A technology for preparing monooctyl itaconic acid and catalysis is applied in the fields of biochemical industry and enzyme catalysis, and can solve the problems of high separation cost, low conversion rate of monoester, many by-products, etc., and achieves high product purity, easy separation, The effect of high conversion rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Preparation of 4-monoctyl itaconate under normal pressure in a solvent system.
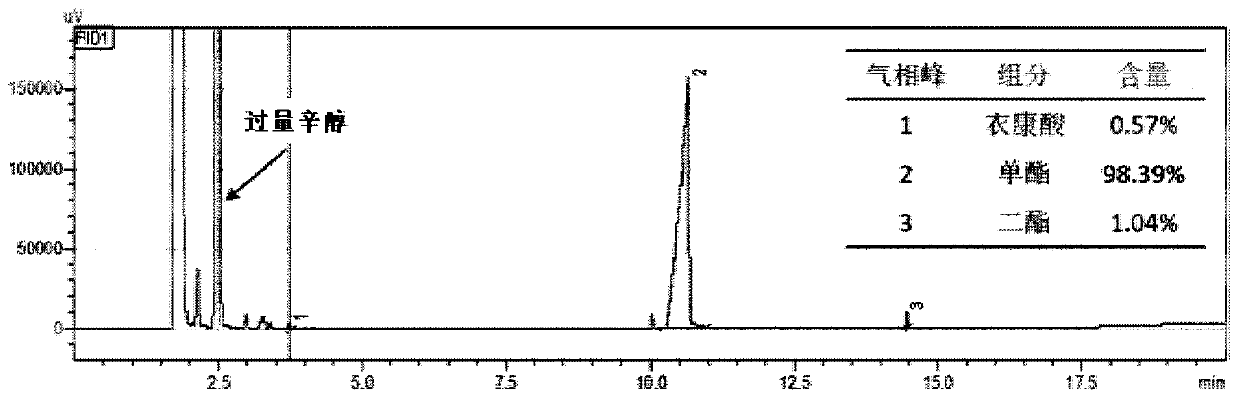
[0096] Step 1. Under normal pressure conditions (1.013×10 5 pa), with 1g itaconic acid (7.69mmol) and 10g (76.9mmol) n-octanol as raw materials, add 0.5g Novozym435 lipase (10000U / g), add solvent toluene 12mL (toluene:n-octanol=1: 1 volume ratio), at 50 DEG C, carry out esterification reaction 24h in 200rpm shaker reactor; The conversion rate of itaconic acid determined by gas chromatography is 99%, and the productive rate of 4-monoctyl itaconate is 98% (see attached image 3 );
[0097] Step 2. After the esterification reaction, remove the lipase by filtering through a solvent filter (0.45 μm nylon filter membrane), pour the reaction solution into a separatory funnel and add saturated saline according to the volume ratio of the reaction solution to saturated saline at 1:1 (Concentration is about 35%), fully mix and shake, let stand for 6h, remove the lower aqueous phase, and c...
Embodiment 2
[0100] Example 2: Preparation of 4-monoctyl itaconate under reduced pressure in a solvent system.
[0101] Step 1. under reduced pressure (1000pa), in there-necked flask, drop into 1g itaconic acid (7.69mmol) and 10g (76.9mmol) n-octanol as raw material, add 0.5g Novozym435 lipase (10000U / g), Add 12 mL of solvent toluene (toluene:n-octanol=1:1 volume ratio), control the water bath at 40°C, and carry out the esterification reaction in a shaker reactor at 200 rpm, connect it to a glass water separator, and carry out the esterification reaction for 12 hours; gas chromatography The conversion rate of itaconic acid was determined to be 99%, and the yield of 4-monoctyl itaconate was 97%;
[0102] Step 2. After the esterification reaction, remove the lipase by filtering through a solvent filter (0.45 μm nylon filter membrane), pour the reaction solution into a separatory funnel and add saturated saline according to the volume ratio of the reaction solution to saturated saline at 1:1 ...
Embodiment 3
[0105] Example 3: Under the solvent system, the packed bed reactor continuously prepared 4-monoctyl itaconate.
[0106] Step 1. Under normal pressure conditions (1.013×10 5 pa), with 5g itaconic acid (7.69mmol) and 50g (76.9mmol) n-octanol as raw materials, add solvent toluene 60mL (toluene:n-octanol=1:1 volume ratio) and mix well, as the reaction substrate;
[0107] Step 2. in long 20cm, inner diameter 1cm, in the steel jacket packed bed reactor of outer diameter 2cm, fill in 2.5g Novozym435 lipase (10000U / g), glass beads are filled in at both ends, by plunger pump The material was pumped into the packed bed reactor from bottom to top, the flow rate was 0.4mL / min, and the jacket temperature was controlled by a circulating water bath at 30°C. When the residence time is 10min; the conversion rate of itaconic acid is 51% as determined by gas chromatography, and the output of 4-monoctyl itaconic acid is 50%; The yield of monooctyl acid was 94%. The total reaction time for all ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com