Mixed ion-electron conductor with garnet structure and application thereof in energy storage device
A technology of mixed ions and electronic conductors, which is applied in the direction of hybrid capacitor electrolytes, electrical components, electrochemical generators, etc., can solve the problems of low interface surface resistance, electronic conductance is not too high, etc., to improve power density, eliminate interface resistance, high lithium The effect of ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
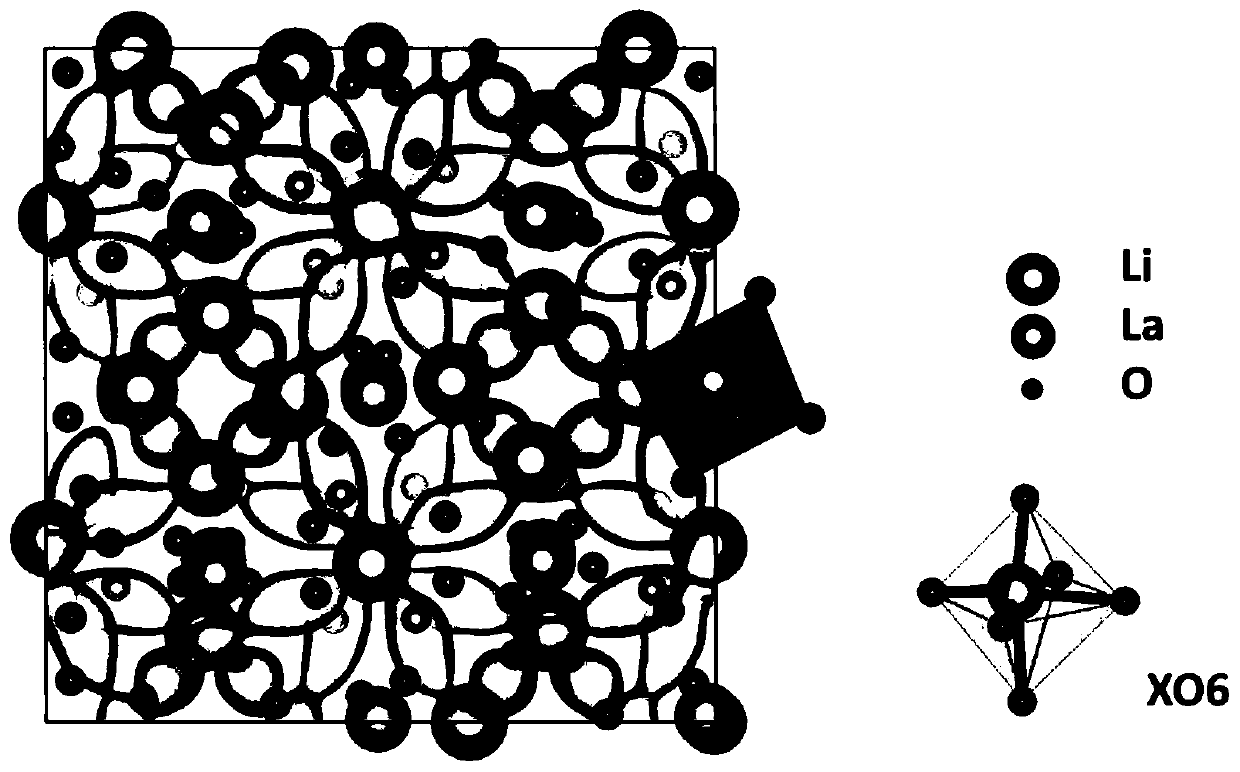
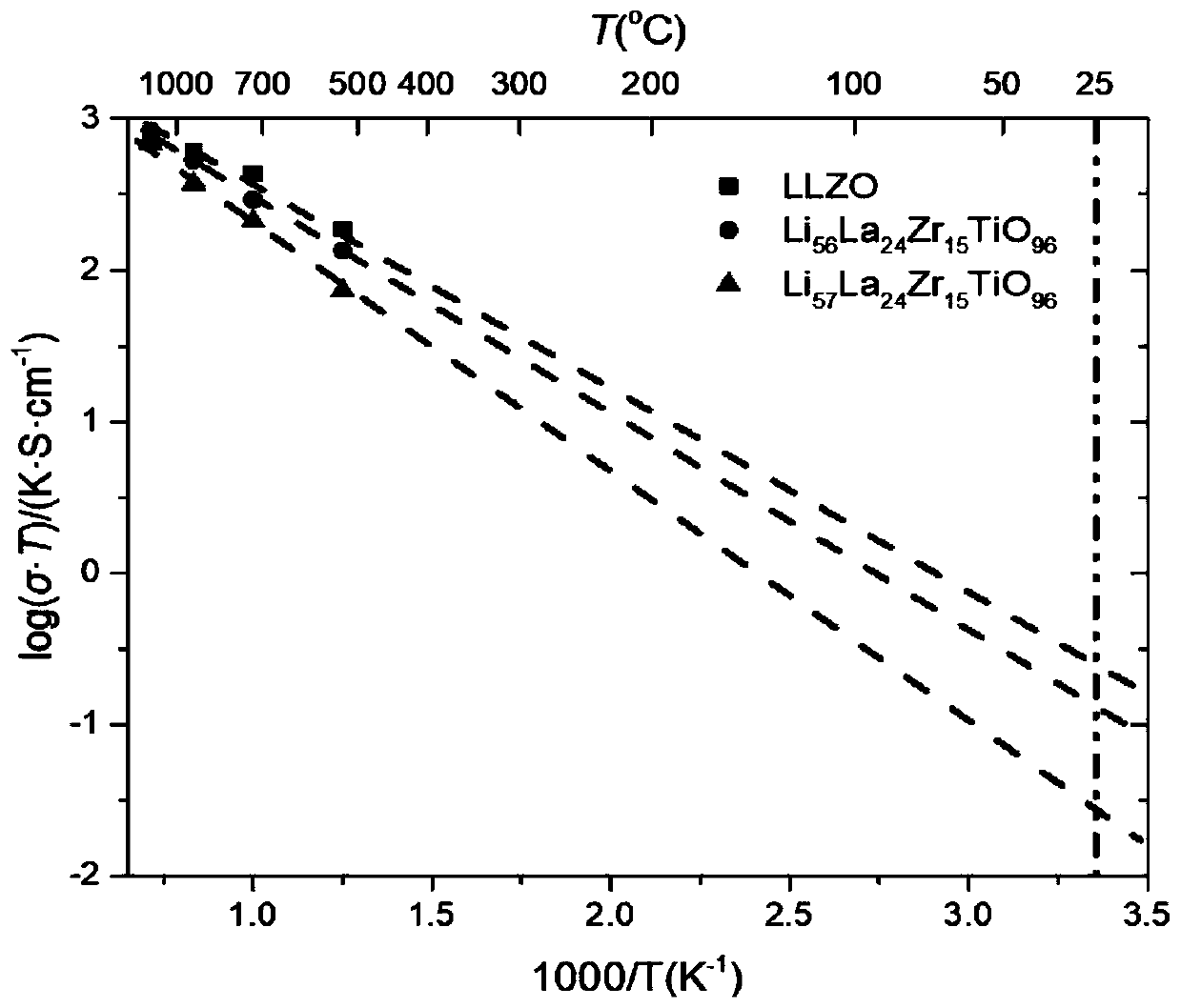
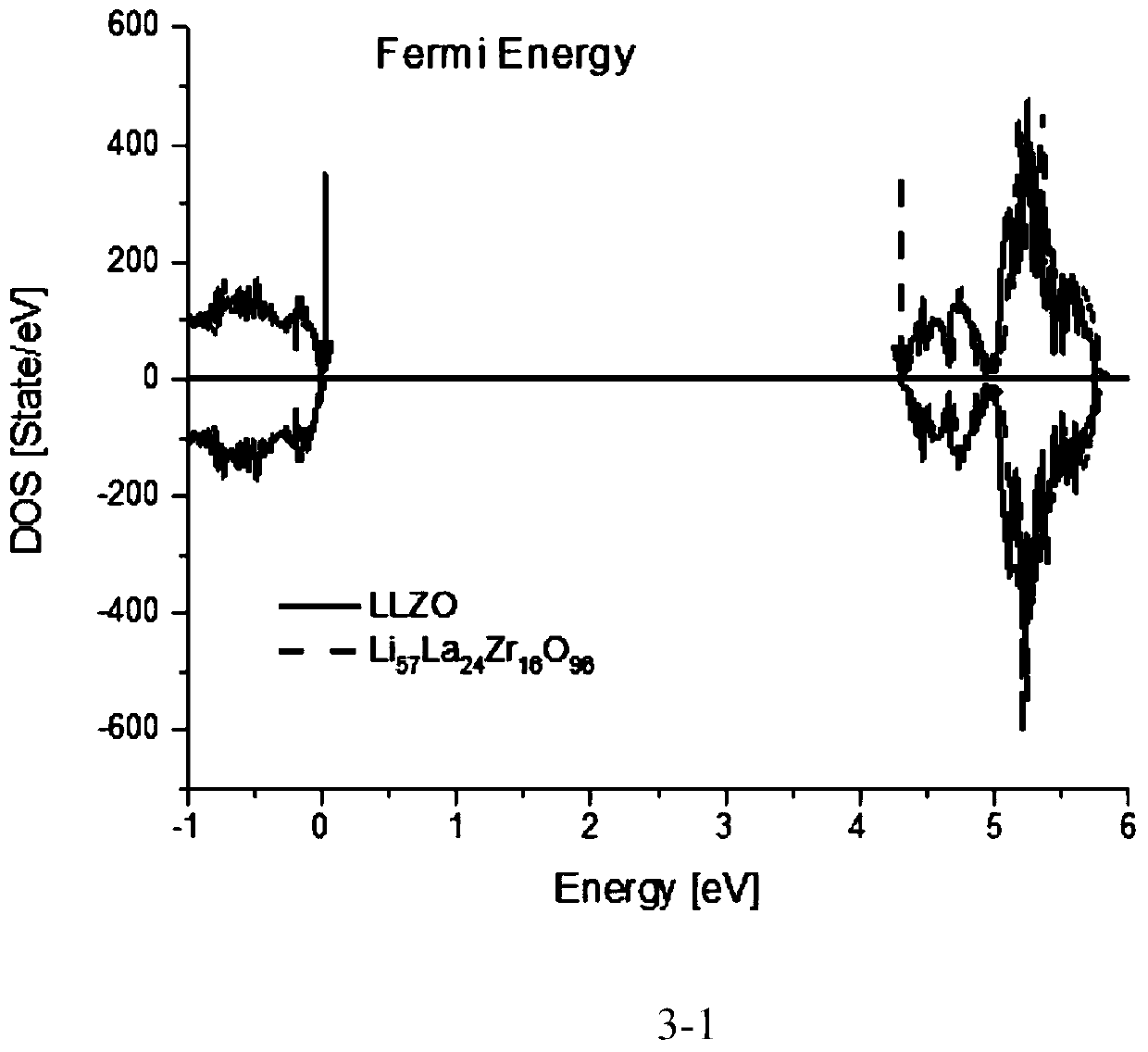
[0056] The mixed ion-electron conductor material of garnet structure refers to the material with figure 1 The stoichiometric ratio of the structure and the doped garnet structure of the non-stoichiometric ratio, and the crystal structure is synthesized by heat treatment (including heating, quenching, annealing), mechanical ball milling, and liquid phase method, and the derived crystalline phase and amorphous phase and a crystalline-amorphous mixed phase. The material system includes a composite phase containing garnet-type mixed conductor materials, including organic and inorganic lithium ion conductors, and electronic conductors / semiconductors. The mixed conductor of the present invention has high ion-electronic conductivity, high stability with electrode and electrolyte materials and small interfacial resistance. The diffusion coefficient of lithium ions determines the ion conductivity, the ion channel and the Arrhenius curve of ion conductivity with temperature as shown in...
Embodiment 2
[0057] Embodiment 2 (Li 6.75 La 3 Zr 1.6875 Ti 0.0625 Ta 0.25 o 12 )
[0058] Anhydrous LiNO 3 ,La(NO 3 ) 3 ·6H 2 O,ZrC 16 h 36 o 4 , TaC 10 h 25 o 5 and TiC 12 h 28 o 4 as raw material, according to Li 6.75 La 3 Zr 1.6875 Ti 0.0625 Ta 0.25 o 12 The stoichiometric ratio of the drug was weighed. TaC 10 h 25 o 5 ,, ZrC 16 h 36 o 4 , TiC 12 h 28 o 4 Disperse in absolute ethanol to obtain solution 1, then La(NO 3 ) 3 ·6H 2 O, LiNO 3 (10wt% excess) was dissolved in deionized water to obtain solution 2; solution 2 was added dropwise into solution 1 to fully hydrolyze the tantalum salt, zirconium salt and titanium salt, and mix evenly with a magnetic stirrer. The obtained gel solution was evaporated to dryness at 150° C., and the obtained dry gel was transferred to an alumina crucible, and treated at 500° C. for 1 hour to remove organic matter. The obtained powder is fully ground, pressed into tablets, covered with mother powder, and calcined at ...
Embodiment 3
[0059] Embodiment 3 (Li 6.7+δ Al 0.1 La 3 Zr 1.75 Ti 0.25 o 12 )
[0060] Take LiOH·H 2 O,La 2 o 3 ,ZrO 2 , TiO 2 and Al(OH) 3 As a raw material, heat (200-500°C) to remove moisture. Mole ratio of raw materials to LiOH·H 2 O:La 2 o 3 :ZrO 2 :TiO 2 :Al(OH) 3 = 6.7:3:1.75:0.25:0.1 mixed with ZrO 2 Balls, ball milled for 1 hour to mix the raw materials evenly. Then the uniformly mixed powder was pressed into tablets, placed in an alumina crucible, covered with mother powder, and calcined in air at 950°C for 12 hours. Grind to obtain a cubic phase powder. Re-press the sheet, cover the mother powder, and sinter at 1100°C for 5 hours to obtain a dense ceramic sheet lithium ion conductor. The ceramic sheet is in contact with metallic lithium, which can intercalate lithium in situ to generate a mixed conductor Li 6.7+δ Al 0.1 La 3 Zr 1.75 Ti 0.25 o 12 (0<δ≤0.25).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com