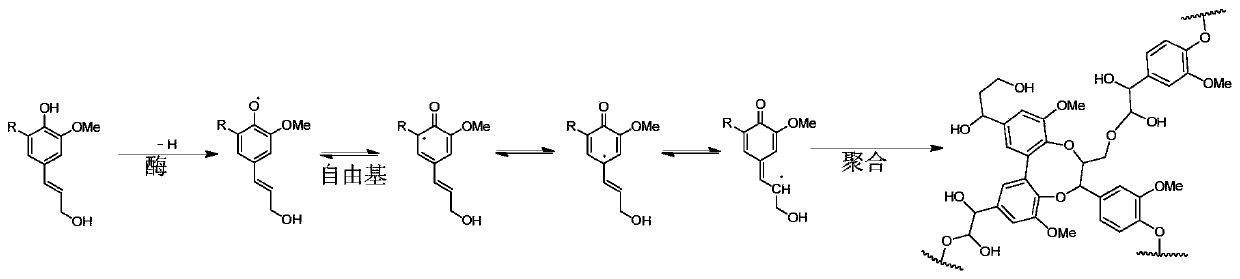
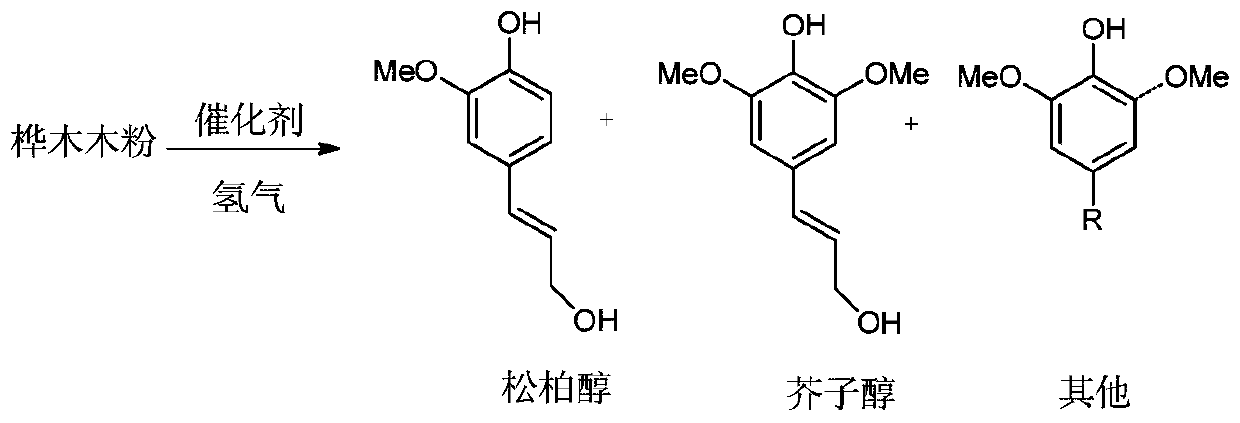
Method for preparing coniferyl alcohol, sinapyl alcohol and derivatives of coniferyl alcohol and sinapyl alcohol by taking lignocellulose as raw material
A technology of lignocellulose and coniferyl alcohol, which is applied in ether preparation, ether separation/purification, organic chemistry and other directions, can solve the problems of unfavorable separation and utilization, complex products, high cost, and achieve resource utilization and high-value utilization, A wide range of sources and high value-added effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] After the birch wood is pulverized to 20-60 meshes, after extracting with toluene and ethanol (2:1, v:v) at 105°C for 12 hours, dewaxed birch wood powder is obtained, and 1 g of the dewaxed birch wood powder is 、MoO 2 / C and methanol in a mass ratio of 1:0.05:20 are added to the high-pressure Parr reactor, and then 3MPa hydrogen is introduced and heated to the reaction temperature (260°C) for 4 hours of reaction at a stirring speed of 800rpm. After the reaction, the temperature is naturally lowered to At room temperature, the pressure was released to normal pressure, and filtered through an organic membrane to obtain a solid residue and a liquid product. The liquid product is extracted with 30mL of dichloromethane, spin-dried by a rotary evaporator at 0.05atm and 45°C, then dissolved and filtered in dichloromethane containing 0.1mg / mL n-tetradecane internal standard, and then enters the GC (gas chromatograph) , GC-MS (gas chromatography-mass spectrometry) for qualitati...
Embodiment 2
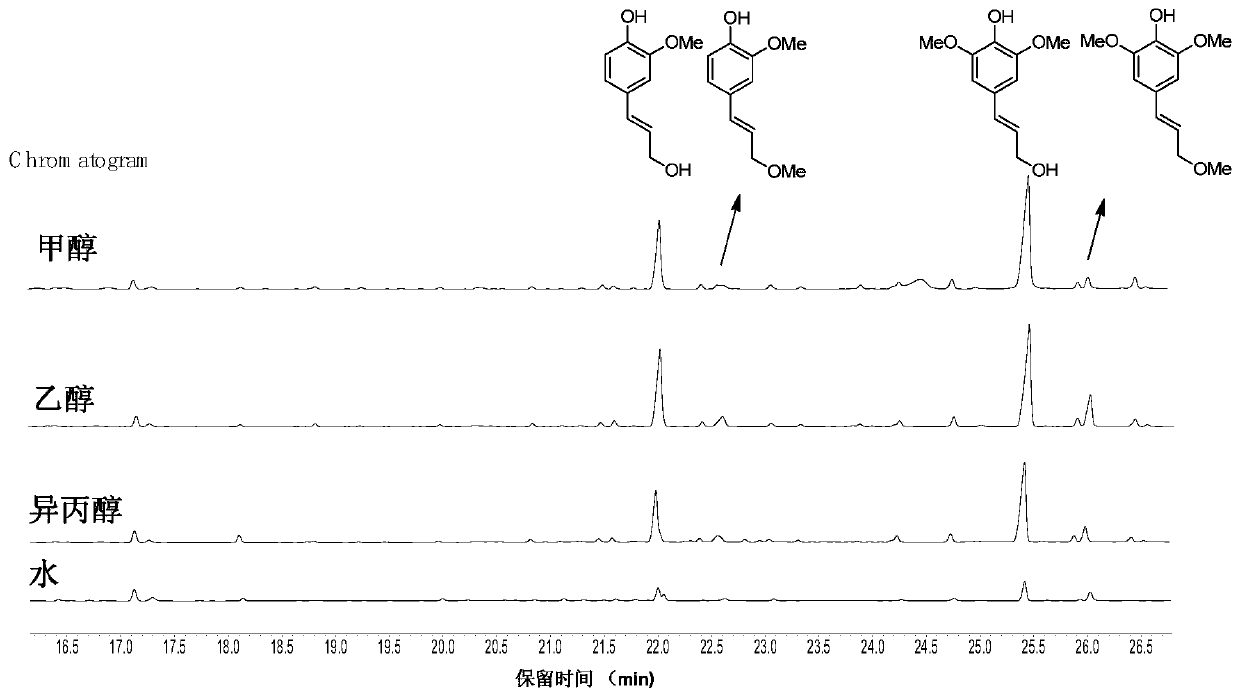
[0047] Same as Example 1, only the reaction solvent of the step is replaced by ethanol, isopropanol or water. Monomer yield, selectivity and separation yield are shown in Table 2, image 3 shown; the table shows MoO in 2 different solvents 2 / C catalyzed hydrogenation results of birch wood flour. From Table 2, image 3 It can be seen from the data that under the organic solvent system, its catalytic degradation lignin monomer yield and the selectivity and separation yield of coniferyl alcohol, sinapyl alcohol and their derivatives have high yields. In this system, lignin cannot be degraded well.
[0048] Table 2 MoO in different solvents 2 Hydrogenation results of / C catalyzed birch wood flour
[0049] solvent Monomer Yield (wt%) Selectivity (wt%) Separation yield (wt%) ethanol 43.6 92 18 Isopropanol 37.8 91 16 water 9.3 - -
Embodiment 3
[0051] Same as Example 1, only the catalyst is recycled, that is, the catalyst collected after the last reaction is activated at high temperature and used. The lignin monomer yield, selectivity and separation yield are shown in Table 3, and Table 3 shows the concentration of MoO in different solvents. 2 / C catalyzed hydrogenation results of birch wood flour. From the data in Table 3, it can be seen that MoO 2 After five cycles of the / C catalyst, the yield of lignin monomer degradation can still reach 43.6%, and the selectivity can still reach more than 90%. This shows that the catalyst has high stability.
[0052] Table 3 MoO in different solvents 2 / C Catalyzed Hydrogenation Results of Birch Wood Flour
[0053]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com