Preparation method of fenbendazole intermediate 2-nitro-4-phenylthioaniline
A technology of phenylthioaniline and fenbendazole, which is applied in the field of preparation of fenbendazole intermediate 2-nitro-4-phenylthioaniline, can solve the problems of high cost, unfavorable industrialization and waste salt generation many problems, to achieve the effect of small waste water discharge, lower production cost and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
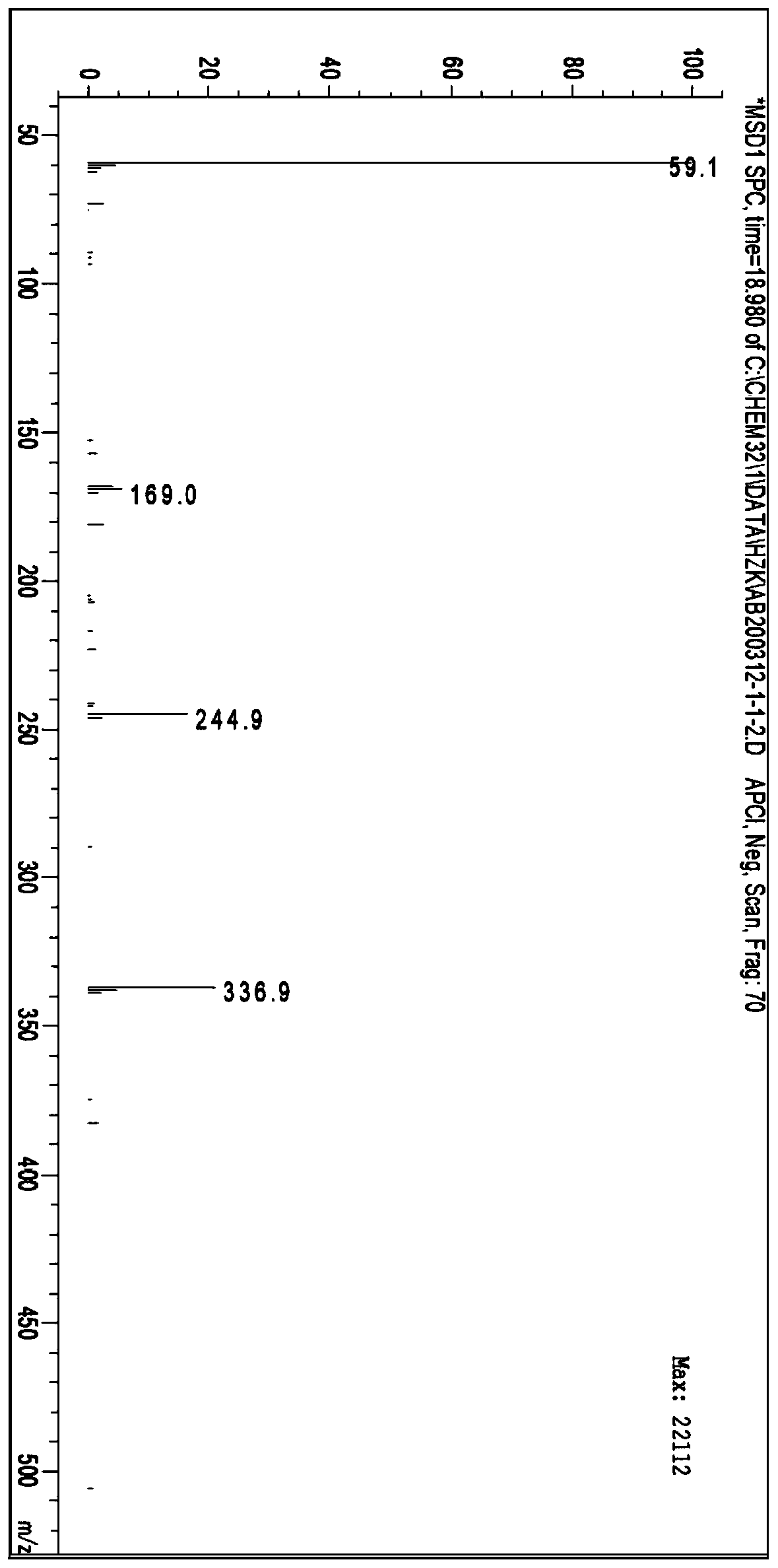
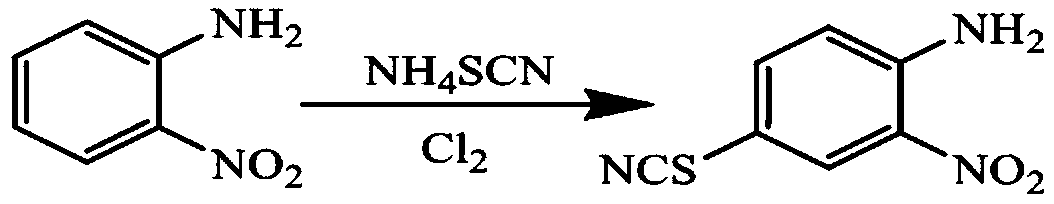
[0031] Add methanol 120g, o-nitroaniline 27.63g, and ammonium thiocyanate 15.99g in a 250ml four-necked bottle, stir evenly, feed chlorine gas to control the reaction temperature at 15°C, and filter to obtain a solid thiocyanation product ( 4-thiocyano-2nitroaniline) 36.88g, yield 94.45%.
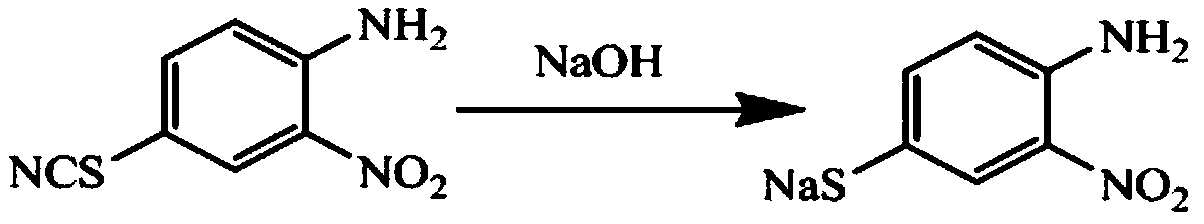
[0032] Add 36.88g of thiocyanation product (4-thiocyano-2nitroaniline) to a 250ml four-necked flask, 100g of purified water, add 8.31g of sodium hydroxide, control the reaction temperature at 35°C, and filter after 20min of reaction An aqueous solution containing 35.59 g of sodium 4-amino-3-nitrobenzenethiophenate was obtained, with a yield of 98.12%;
[0033] 4-amino-3-nitrobenzene sodium aqueous solution is warmed up to 60 DEG C, drips the ethanol solution that contains 29.10g bromobenzene, adds extraction agent toluene (toluene according to its and 4-amino-3-nitrobenzene Sodium sulfur aqueous solution was added in a mass ratio of 1:1), and stirred for 10 min to obtain a toluene solution...
Embodiment 2
[0035] Add 120g of methanol, 27.63g of o-nitroaniline, and 15.99g of ammonium thiocyanate in a 250ml four-necked bottle, stir evenly, feed chlorine gas, control the reaction temperature at 18°C, and filter to obtain the thiocyanation product (4 -thiocyano-2 nitroaniline) 36.77g, yield 94.15%.
[0036] In a 250ml four-necked flask, add 36.77g of thiocyanation product (4-thiocyano-2nitroaniline), 100g of methanol, add 8.29g of sodium hydroxide, control the reaction temperature at 38°C, and filter after 20 minutes of reaction to obtain An alcohol solution containing 35.42 g of sodium 4-amino-3-nitrobenzenethiophenate, with a yield of 97.98%;
[0037] Raise the temperature of the 4-amino-3-nitrobenzenethiosodium alcohol solution to 62°C, add dropwise an ethanol solution containing 34.76g of bromobenzene, add water (according to its mass ratio to toluene at 1:1) and extract Agent toluene (toluene is added according to the ratio of 1:1 of its mass ratio with 4-amino-3-nitrophenylth...
Embodiment 3
[0039] Add 120g of methanol, 27.63g of o-nitroaniline, and 15.99g of ammonium thiocyanate in a 250ml four-necked bottle, stir evenly, feed chlorine, control the reaction temperature at 20°C, and filter to obtain the thiocyanation product (4 -thiocyano-2nitroaniline) 36.71g, yield 94.01%.
[0040] Add thiocyanation product (4-thiocyano-2 nitroaniline) 36.71g in 250ml four-necked bottle, 100g volume concentration is the ethanol of 60%, add sodium hydroxide 9.02g, control reaction temperature is 40 ℃, through After 20 minutes of reaction, an alcoholic solution containing 35.69 g of sodium 4-amino-3-nitrobenzenethiophenate was obtained by filtration, with a yield of 98.87%;
[0041] Raise the temperature of the 4-amino-3-nitrobenzenethiosodium alcohol solution to 65°C, add dropwise an ethanol solution containing 35.02g of bromobenzene, add water (according to its mass ratio to toluene at 1:1) and extract Agent toluene (toluene is added according to the ratio of 1:1 of its mass ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com