Organic small-molecule semiconductor material containing rhodanine condensed (similar) isatin as well as preparation method and application of organic small-molecule semiconductor material
A rhodanine and small molecule technology, which is applied in the field of organic small molecule semiconductor materials containing rhodanine fused isatin and its preparation, can solve problems such as poor stability, achieve enhanced stability, simple preparation method, and molecular structure novel effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Add isatin (1equiv) and potassium carbonate (2.5equiv) to the reaction flask, add anhydrous DMF and tetrahydrofuran after ventilation, slowly add alkyl bromide to the flask, and react at 50°C after dripping overnight. After extraction with dichloromethane and water, the organic phases were combined and dried over anhydrous magnesium sulfate. After spin-drying dichloromethane, the product was separated and purified by column chromatography to obtain a yellow solid product. The H NMR and C NMR spectra of 6-bromoisatin are consistent with the literature (Li, J.-L.; Chai, Y.-F.; Wang, W.V.; Shi, Z.-F.; Xu, Z.-G .; Zhang, H.-L., Chem. Commun. 2017, 53, 5882. Luo, X.; Tran, D.T.; Kadlubowski, N.M.; ., Macromolecules, 2018, 51, 8486.)
[0045]The preparation of 6-bromo-1-methylindoline-2,3-dione, the reaction formula is as follows:
[0046]
[0047] The preparation of 6-bromo-1-butylindoline-2,3-dione, the reaction formula is as follows:
[0048]
[0049] The prepar...
Embodiment 2
[0058] Add four-p-hexylbenzene-indaprodithiophene bistin (1equiv) and 6-bromoisatin (2.5equiv) in the reaction flask, add ultra-dry toluene and catalyst Pd (PPh 3 ) 4 (5% equiv), heated to 125°C to react overnight. After cooling to room temperature, it was extracted with chloroform and water. The organic phases were combined and dried with anhydrous magnesium sulfate, evaporated to dryness and purified by column chromatography to obtain a black solid product. All the obtained compounds were verified as target products by H NMR and C NMR spectra.
[0059] When isatin is 6-bromo-1 methylindoline-2,3-dione, the reaction formula is as follows:
[0060]
[0061] When isatin is 6-bromo-1-octyl indoline-2,3-dione, the reaction formula is as follows:
[0062]
[0063] When isatin is 6-bromo-5-fluoro-1-octylindoline-2,3-dione, the reaction formula is as follows:
[0064]
[0065] When isatin is 6-bromo-5-fluoro-1-octylindoline-2,3-dione, the reaction formula is as follows...
Embodiment 3
[0072] Add the three compound intermediates (1equiv) and (alkyl) rhodanine (5equiv) obtained in Example 2 in the reaction flask, add chloroform after pumping, add a few drops of triethylamine after stirring for 5 minutes, room temperature React overnight. Extract with chloroform and water, combine the organic phases, and dry the organic phases with anhydrous magnesium sulfate. After evaporating chloroform to dryness, the product was purified by column chromatography to obtain a green solid product.
[0073]
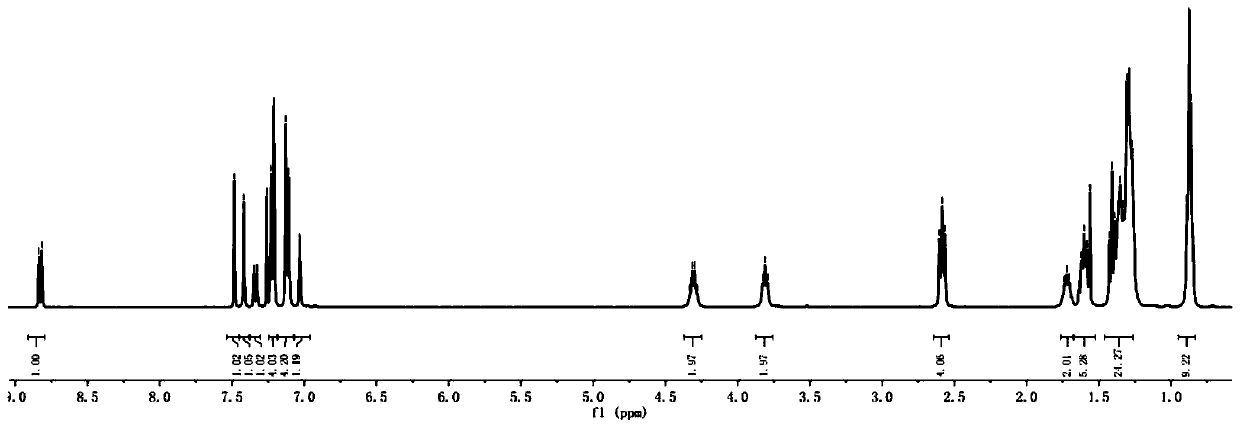
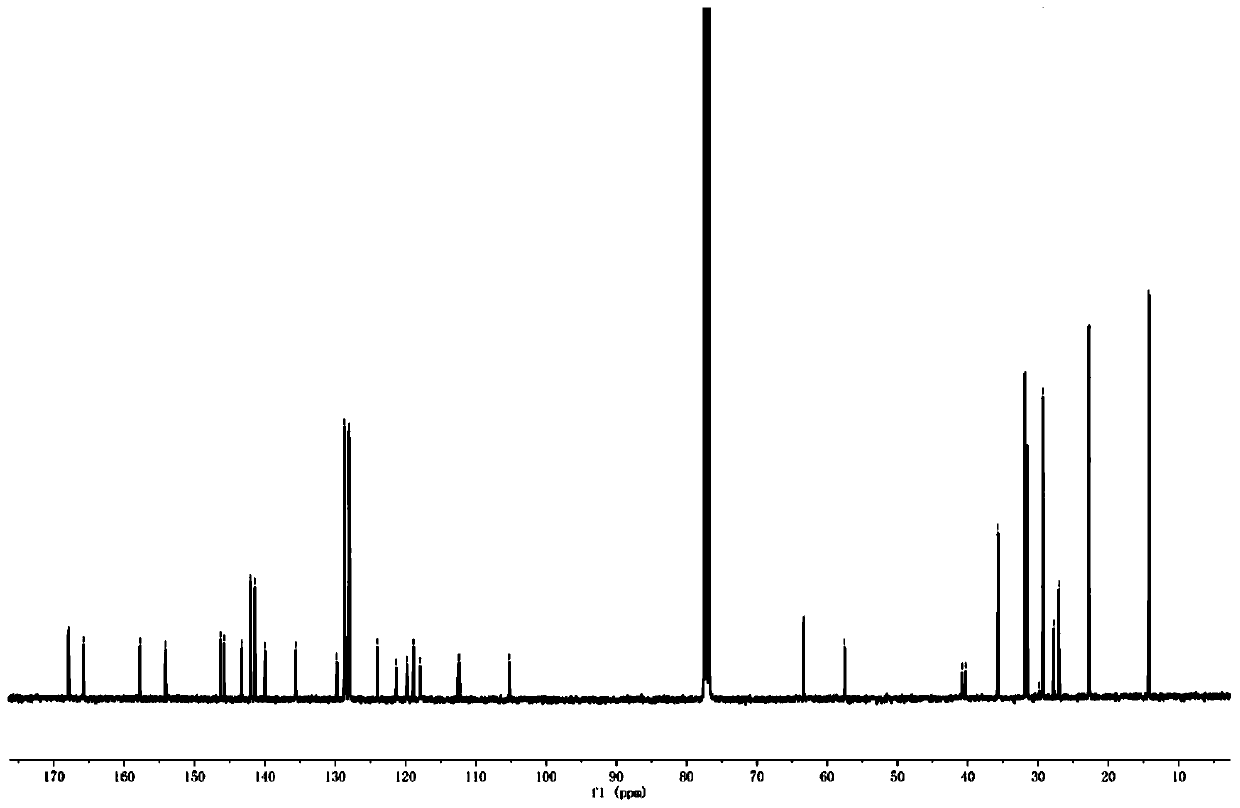
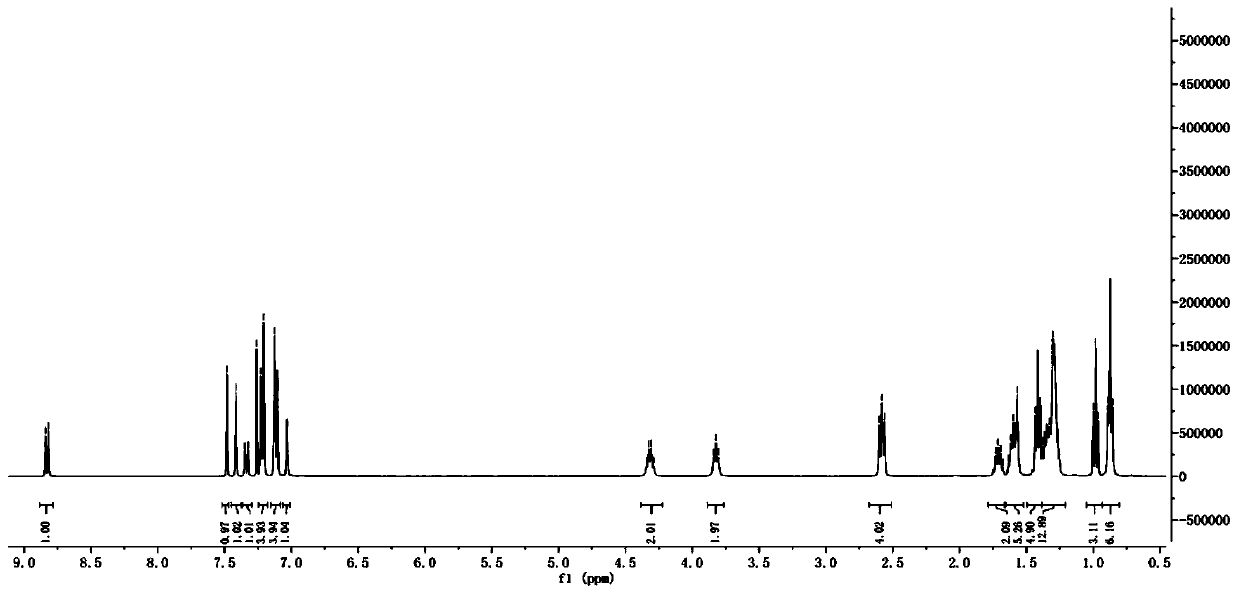
[0074] The H NMR and C NMR spectra of compound WH7 are characterized as follows:
[0075] 1 H NMR (400MHz, CDCl 3 ,298K):8.83(s,1H),7.48(d,1H),7.41(s,1H),7.35(d,1H),7.23(d,4H),7.13(d,4H),7.03(s, 1H), 4.33(q, 2H), 3.81(t, 2H), 2.58(t, 4H), 1.72(m, 2H), 1.60(t, 4H), 1.45-1.19(m, 25H), 0.84(m ,9H). 13 C NMR (100MHz, CDCl 3 ,298K):182.18,159.05,157.80,154.20,151.91,145.52,144.59,144.04,142.17,141.24,135.61,128.70,127.96,122.23,120.08,118.07,116.23,106.06,63.37,40.37...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com