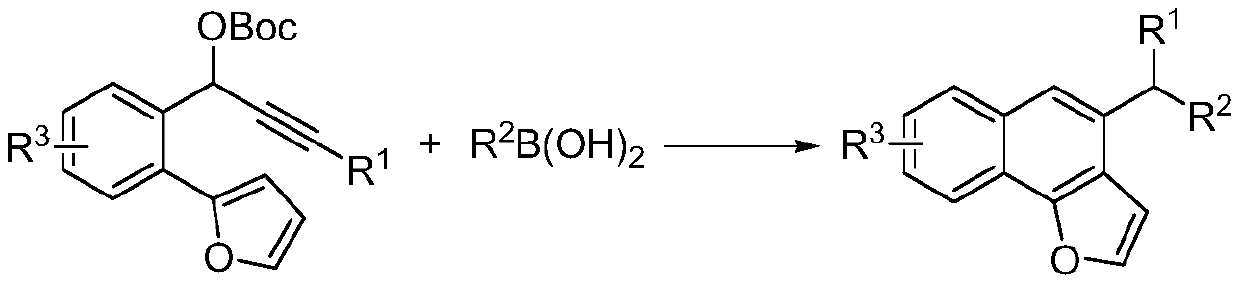Synthesis method of naphthofuran derivative, naphthofuran derivative and application
A synthetic method and derivative technology, applied in drug combination, antineoplastic drugs, organic chemistry, etc., can solve the problems of poor functional group tolerance, high cost of raw materials, cumbersome post-processing, etc., and achieve good yield, simple raw materials, and post-processing simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
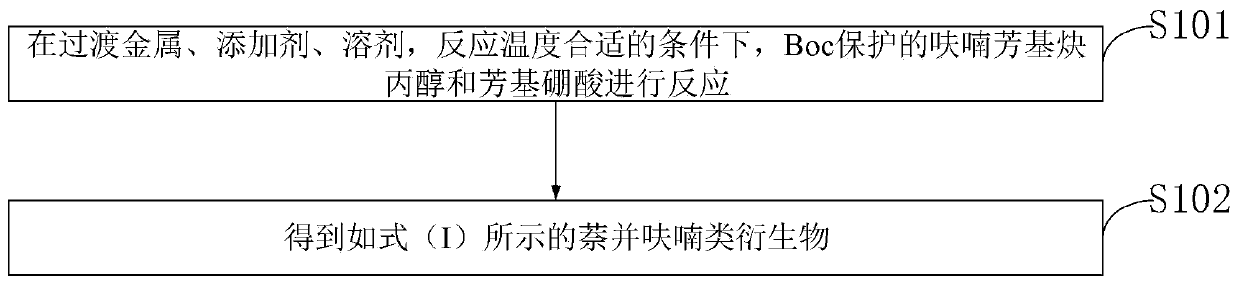
[0050] Such as figure 1 As shown, the embodiment of the present invention also proposes a synthetic method of naphthofuran derivatives as shown in formula (I), including:
[0051] S101, under the conditions of transition metal, additive, solvent, and reaction temperature, Boc-protected furyl aryl propargyl alcohol and aryl boronic acid are reacted.
[0052] S102, obtaining naphthofuran derivatives represented by formula (I).
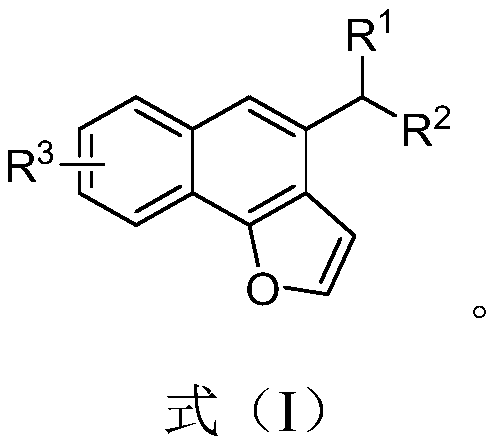
[0053] In step S10, o-furyl aryl propargyl alcohol derivatives and aryl boronic acid are used as raw materials.
[0054] Its reaction process is shown in formula (II):
[0055]
[0056] where, where, R 1 , R 2 Respectively selected from phenyl, naphthyl, aryl substituted by electron-donating group, aryl substituted by electron-withdrawing group, alkyl, hydrogen; R 3 For halogen, methoxy, hydrogen.
[0057] In the embodiment of the present invention, R 1 , R 2 Respectively selected from phenyl, naphthyl, p-methylphenyl, p-methoxyphenyl, p-chlor...
Embodiment 1
[0074] Embodiment 1: the synthesis of IA
[0075]
[0076] o-furyl aryl propargyl alcohol derivatives, aryl boronic acid, catalyst, additive, and reaction solvent are respectively Boc-protected o-furan phenyl phenyl propargyl alcohol, phenylboronic acid, tetrakistriphenylphosphine palladium, cesium carbonate, 1, 4-dioxane. The dosages are 0.3mmol of Boc-protected o-furylphenylphenylpropargyl alcohol, 0.6mmol of phenylboronic acid, 0.006mmol of tetrakistriphenylphosphine palladium, 0.6mmol of cesium carbonate, and 3mL of 1,4-dioxane. React at 100°C for 2 hours, then cool to room temperature, concentrate, and perform column chromatography to obtain the target product formula (IA), a light yellow solid, with an isolation yield of 78%. Mp 194-196°C.
[0077] NMR data: 1 H NMR (CDCl 3 ,400MHz):δ5.96(s,1H),5.60(d,J=1.6Hz,1H),7.17(s,1H),7.25(d,J=6.0Hz,4H),7.30(t,J= 6.0Hz, 2H), 7.36(t, J=5.6Hz, 4H), 7.47(t, J=5.6Hz, 1H), 7.59(t, J=5.6Hz, 1H), 7.67(d, J=1.6Hz ,1H),7.80(d,J=6.4...
Embodiment 2
[0079] Embodiment 2: the synthesis of IB
[0080]
[0081] Chlorine-substituted o-furyl aryl propargyl alcohol derivatives, aryl boronic acid, catalyst, additive, and reaction solvent are respectively Boc-protected o-furyl phenyl phenyl propargyl alcohol, phenylboronic acid, tetrakistriphenylphosphine palladium, cesium carbonate, 1,4-Dioxane. The amounts used are respectively 0.3 mmol of Boc-protected chlorine-substituted o-furylphenylphenyl phenyl propargyl alcohol, 0.6 mmol of phenylboronic acid, 0.006 mmol of tetrakistriphenylphosphine palladium, 0.6 mmol of cesium carbonate, and 3 mL of 1,4-dioxane. React at 100°C for 2 hours, then cool to room temperature, concentrate, and perform column chromatography to obtain the target product formula (IB), a pale yellow solid, with an isolation yield of 72%. Mp 171-173°C.
[0082] NMR data: 1 H NMR (CDCl 3 ,400MHz):δ5.88(s,1H),6.53(s,1H),7.07(s,1H),7.17-7.34(m,11H),7.60-7.67(m,2H),8.23(s,1H ). 13 C NMR (CDCl 3 ,100MHz): δ54...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Separation yield | aaaaa | aaaaa |
| Separation yield | aaaaa | aaaaa |
| Separation yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com